最小金属酶模型中金属配位和反应性的DFT研究
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
金属酶通过结构约束精确控制其金属配位环境来实现催化功能。然而,结构刚度对金属取代的影响及其对酶结构和反应性的影响尚未完全阐明。为了解决这个问题,我们使用DFT研究了结构约束如何影响人类碳酸酐酶II (CA II)活性位点内的金属配位几何、能量学和反应性。我们从含有Zn2+(原生)、Cu2+、Ni2+和Co2+的x射线晶体结构中构建了金属取代CA II的半约束模型。构建半约束模型来模拟蛋白质活性位点的微环境,并对多种DFT方法进行基准测试,以确定准确高效的计算方法。结构约束导致了具有多个局部最小值的崎岖能源景观,我们发现在金属替代时,结构约束和内在配位化学之间的竞争导致了最终几何形状的不同后果。我们还发现,金属酶CA II中的原生金属离子(Zn2+)在测试的金属离子中并不总是表现出最强的结合;相反,趋势遵循欧文-威廉姆斯系列。然而,亲电性分析表明,受约束的几何形状调节了金属中心的电子反应性,Zn2+始终表现出最高的亲电性,这解释了金属酶的进化优化。这些发现增强了我们对结构约束下金属配位的理解,并为探索人工金属酶中的金属取代提供了计算基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
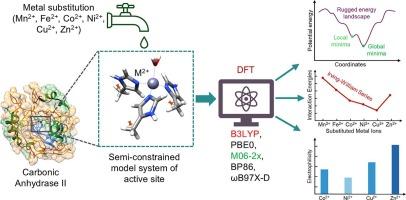
DFT investigation of metal coordination and reactivity in minimal metalloenzyme models
Metalloenzymes achieve catalytic functionality by precisely controlling their metal coordination environments through structural constraints. However, the influence of structural rigidity on metal substitution and its impact on enzyme structure and reactivity has not been fully elucidated. To address this, we investigated how structural constraints affect metal coordination geometry, energetics, and reactivity within the active site of human carbonic anhydrase II (CA II) using DFT. We constructed semi-constrained models of metal substituted CA II from their X-ray crystal structures containing Zn2+ (native), Cu2+, Ni2+, and Co2+. Semi-constrained models were constructed to mimic the microenvironment of the protein active site, and multiple DFT methods were benchmarked to identify an accurate and efficient computational approach. Structural constraints lead to a rugged energy landscape with multiple local minima, and we found that upon metal substitutions, the competition between the structural constraints and the intrinsic coordination chemistry leads to diverse consequences in final geometry. We also found that the native metal ion (Zn2+) in metalloenzymes CA II does not always exhibit the strongest binding among the metal ions tested; instead, the trends follow the Irving-Williams series. However, electrophilicity analysis revealed that constrained geometries modulate the electronic reactivity of the metal center, with Zn2+ consistently exhibiting the highest electrophilicity, and this explains the evolutionary optimization of the metalloenzyme. These findings enhance our understanding of metal coordination under structural constraints and provide a computational basis for exploring metal substitutions in artificial metalloenzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


