调节HF/吡啶介质的酸度以控制烯烃的化学发散氟和氢杂烷基化。
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氧羰基离子是一种重要的反应中间体,在有机合成中有着广泛的应用。其中,原生氧羰基离子的研究较少,部分原因是在标准条件下难以控制其反应性。在这里,我们表明,这些物种的反应性可以在强酸性HF/吡啶介质中精细调节。通过利用HF/吡啶溶液的可调酸度,它们与烯烃的反应可以转向氟化或氢氧烷基化。额外的光谱和计算研究提供了对机制的深入了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
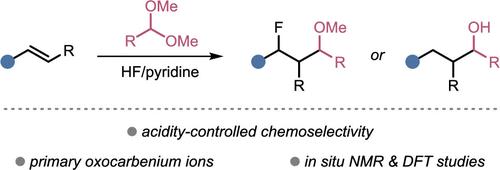
Regulating the Acidity of HF/Pyridine Media to Control the Chemodivergent Fluoro- and Hydro-Heteroalkylation of Olefins
Oxocarbenium ions are important reactive intermediates with many applications in organic synthesis. Among them, primary oxocarbenium ions have been much less explored, in part due to the difficulty of controlling their reactivity under standard conditions. Here, we show that the reactivity of such species can be finely modulated in strongly acidic HF/pyridine media. By leveraging the tunable acidity of HF/pyridine solutions, their reaction with olefins can be diverted toward either fluoro- or hydro-oxyalkylation. Additional spectroscopic and computational investigations provide insights into the mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


