改良的l -天冬酰胺酶变体具有增强的治疗特性,以改善儿童急性淋巴白血病的治疗。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
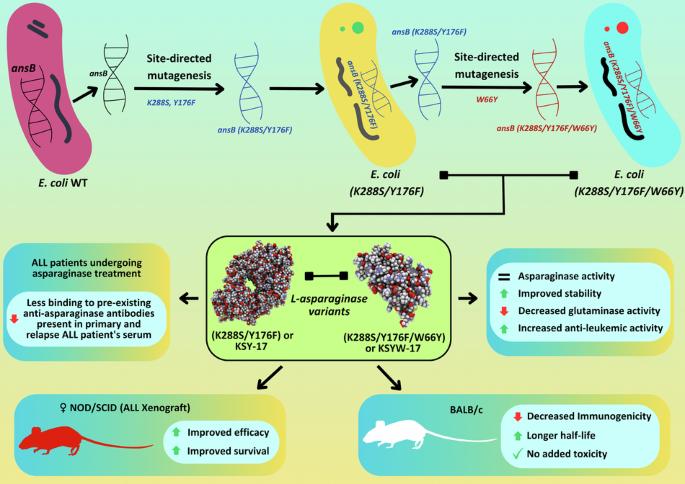
大肠杆菌l -天冬酰胺酶(EcA)是一种多药急性淋巴白血病(ALL)治疗方案的关键组成部分,它的一些局限性降低了其治疗效果。主要的缺点包括免疫原性、血清不稳定、半衰期较短以及伴随的谷氨酰胺酶活性导致神经毒性和胰腺炎。聚乙二醇化天冬酰胺酶和Erwinase具有较好的治疗潜力,但价格昂贵。利用定点诱变技术,我们通过替换二聚体界面和b细胞表位区域的特定氨基酸残基,创造了几种EcA变体。经过多轮筛选和筛选,我们鉴定出两个EcA变异,即K288S/Y176F (KSY-17)和K288S/Y176F/W66Y (KSYW-17),它们的天冬酰胺酶活性与野生型(WT)相当,谷氨酰胺酶活性显著低于野生型(30.36 U/mg,而KSY-17和KSYW-17分别为1.54和0.99 U/mg)。KSYW-17的免疫原性比WT低,IgG和IgM的应答分别低4.8-5.3倍和2.4-3.8倍。与WT EcA相比,我们还观察到这些变体与ALL患者血清中已有抗体的结合明显减少(约1.5-2倍)。药代动力学研究表明,KSYW-17(213.3±6.5 min)和KSYW-17(244.8±35.5 min)的血浆半衰期比WT(101.1±5.1 min)更长。两种变体在小鼠中高达5000 IU/kg(单次剂量)和1600 IU/kg(重复剂量)均无毒性。所有异种移植小鼠研究显示,KSY-17和KSYW-17给药小鼠白血病负担分别减少90%和70%,而WT重复给药后为30%,同时小鼠存活率显著提高(KSY-17 100% vs. 70% vs. KSYW-17 vs. WT 10%)。总体而言,经工程修饰的EcA变体显示出更好的治疗效果,因此使它们成为原发性和复发性ALL治疗的有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineered L-asparaginase variants with enhanced therapeutic properties to improve treatment of childhood acute lymphatic leukemia
Escherichia coli L-asparaginase (EcA), a key component of a multi-drug acute lymphatic leukemia (ALL) treatment regimen, has several limitations that reduce its therapeutic efficacy. The major disadvantages include immunogenicity, serum instability, shorter half-life, and accompanying glutaminase activity that causes neurotoxicity and pancreatitis. Pegylated asparaginase and Erwinase have better therapeutic potential, but they are expensive. Using site-directed mutagenesis, we created several EcA variants by substituting specific amino acid residues at the dimer-dimer interface and B-cell epitope regions. After several rounds of screening and selection, we identified two EcA variants viz. K288S/Y176F (KSY-17) and K288S/Y176F/W66Y (KSYW-17), which showed comparable asparaginase activity to wild-type (WT) and significantly less glutaminase activity (30.36 U/mg for WT vs 1.54 and 0.99 U/mg for KSY-17 and KSYW-17). KSYW-17 was less immunogenic than WT, eliciting 4.8–5.3-fold and 2.4–3.8-fold less IgG and IgM responses, respectively. Compared to WT EcA, we also observed significantly less (~1.5-2-fold) binding of these variants to pre-existing antibodies in ALL patients’ serum. Pharmacokinetic studies showed that KSY-17 (213.3 ± 6.5 min) and KSYW-17 (244.8 ± 35.5 min) had longer plasma half-lives than WT (101.1 ± 5.1 min). Both variants showed no toxicity up to 5000 IU/kg (single dose) and 1600 IU/kg (repeat dose) in mice. ALL xenograft mice studies showed a 90% and 70% reduction in leukemia burden in KSY-17 and KSYW-17 administered mice, respectively, as compared to 30% for WT after repeat dose administration, accompanied by significantly higher mice survival (100% vs. 70% vs. 10% for KSY-17 vs. KSYW-17 vs. WT). Overall, the engineered EcA variants’ showed improved therapeutic efficacy, thus making them promising candidates for primary and relapsed ALL treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


