来源依赖的代谢变异:白术提取物的化学多样性如何导致模拟消化的阶段特异性变化
IF 3.1
3区 医学
Q2 CHEMISTRY, ANALYTICAL
Journal of pharmaceutical and biomedical analysis
Pub Date : 2025-07-25
DOI:10.1016/j.jpba.2025.117082
引用次数: 0
摘要
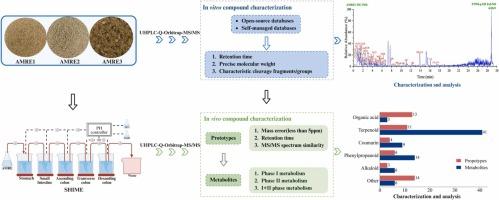
苍术是一种中药,因其具有抗炎、抗肿瘤、调节胃肠等药理作用而被广泛应用于临床。然而,由于中药提取物的复杂性质,目前关于不同来源苍术提取物(AMRE)成分变化对胃肠代谢过程影响的研究很少。本研究利用超高效液相色谱-四极杆-轨道阱串联质谱(UHPLC-Q-Orbitrap-MS/MS)和人类肠道微生物生态系统模拟器(SHIME)建立了一种体外和体内综合化合物分析策略,研究了不同来源的AMRE化学成分变化引起的代谢变化。共发现117种化学成分,主要分类为萜类、有机酸类、生物碱类、香豆素类和苯丙素类。鉴定出51种原型成分和79种代谢物。代谢过程主要在萜类化合物中观察到,其反应类型包括羟基化,氧化,氢化,甲基化,葡萄糖醛酸化和磺化。动态变化分析显示,大多数原型成分在结肠中显著减少,而代谢物在小肠和结肠中均显著富集。差异分析表明,AMRE3萜类化合物含量最高,AMRE1化学成分平均含量最高,AMRE2化学成分平均含量最低。这些差异在原型成分和代谢行为中都得到了一致的观察,从而证实了代谢物分布与化学成分之间的强大相关性。本研究首次阐明了不同来源AMRE化学成分的变化及其在人体胃肠道中的代谢特征和差异,为进一步阐明其药理作用和临床应用的物质基础提供了可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Origin-dependent metabolic variations: How Atractylodes macrocephalae Rhizoma extract’s chemical diversity leads to stage-specific changes in simulated digestion
Atractylodes macrocephalae Rhizoma (AMR), a traditional Chinese medicine, is extensively utilized in clinical practice for its pharmacological properties, including anti-inflammatory, anti-tumor, and gastrointestinal regulatory effects. Nonetheless, the intricate nature of traditional Chinese medicine extracts has resulted in few studies into the effects of compositional variations in Atractylodes macrocephalae Rhizoma extracts (AMRE) from diverse sources on gastrointestinal metabolic processes. This study developed an integrated in vitro and in vivo compound analysis strategy utilizing Ultrahigh-performance liquid chromatography Quadrupole-Orbitrap tandem mass spectrometry (UHPLC-Q-Orbitrap-MS/MS) and the Simulator of Human Intestinal Microbial Ecosystem (SHIME) to examine the metabolic alterations caused by variations in the chemical constituents of AMRE from diverse sources. A total of 117 chemical constituents were found, primarily classified as terpenoids, organic acids, alkaloids, coumarins, and phenylpropanoids. 51 prototype components and 79 metabolites were identified. The metabolic processes were predominantly observed among terpenoids, with reaction types encompassing hydroxylation, oxidation, hydrogenation, methylation, glucuronidation, and sulfonation. Analysis of dynamic changes revealed that the majority of the prototype components underwent a considerable reduction in the colon, while the metabolites were markedly enriched in both the small intestine and colon. Differential analysis showed that AMRE3 contained the highest number of terpenoid compounds, AMRE1 exhibited the highest average content of chemical constituents, and AMRE2 had the lowest. These disparities were consistently observed in both prototype components and metabolic behaviors, thereby affirming the robust correlation between metabolite distribution and chemical constituents. This study elucidates, for the first time, the variations in the chemical constituents of AMRE from diverse sources and the metabolic characteristics and discrepancies they elicit in the human gastrointestinal tract (GI tract), offering a viable strategy for further clarifying the material basis of its pharmacological effects and clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.70
自引率
5.90%
发文量
588
审稿时长
37 days
期刊介绍:
This journal is an international medium directed towards the needs of academic, clinical, government and industrial analysis by publishing original research reports and critical reviews on pharmaceutical and biomedical analysis. It covers the interdisciplinary aspects of analysis in the pharmaceutical, biomedical and clinical sciences, including developments in analytical methodology, instrumentation, computation and interpretation. Submissions on novel applications focusing on drug purity and stability studies, pharmacokinetics, therapeutic monitoring, metabolic profiling; drug-related aspects of analytical biochemistry and forensic toxicology; quality assurance in the pharmaceutical industry are also welcome.
Studies from areas of well established and poorly selective methods, such as UV-VIS spectrophotometry (including derivative and multi-wavelength measurements), basic electroanalytical (potentiometric, polarographic and voltammetric) methods, fluorimetry, flow-injection analysis, etc. are accepted for publication in exceptional cases only, if a unique and substantial advantage over presently known systems is demonstrated. The same applies to the assay of simple drug formulations by any kind of methods and the determination of drugs in biological samples based merely on spiked samples. Drug purity/stability studies should contain information on the structure elucidation of the impurities/degradants.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


