白色念珠菌metacaspase n端在钙结合中的作用。
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2025-07-28
DOI:10.1016/j.bbapap.2025.141090
引用次数: 0
摘要
metacaspase是CD家族的成员,与哺乳动物caspase具有结构相似性,但具有独特的特征。本研究深入研究了白色念珠菌metacaspase CaMCA-Ia,一种I型metacaspase。CaMCA-Ia表现出Ca2+依赖性的自动加工,并呈现一个疏水的n端,这与II型metacaspase不同。截断的CaMCA-Ia (CaMCA-Ia-ΔN86)缺少86个n端氨基酸,经过逐渐的自我加工和分子间加工。添加钙诱导多步骤加工,导致成熟。像哺乳动物的半胱天冬酶一样,CaMCA-Ia-ΔN86可以激活其他分子,表明分子间的激活和加速成熟。与Ca2+相互作用的CaMCA-Ia全长和截短形式的不同结合位点强调了n端在改变钙亲和力中的作用。这些发现增强了对metacaspase复杂的激活和成熟动力学的理解,为致病真菌的潜在药物靶点提供了见解。CaMCA突变体D252A、D268A、D299A和D268/269 A对钙表现出不同的反应,而相应的CaMCA- ia -ΔN86突变体表现出不同的加工模式。D268/299 A突变体与钙孵育48小时后出现加工。CaMCA结构和功能的改变,如n端缺失和钙结合位点天冬氨酸的变化,为CaMCA酶活性和自动加工是如何被调节提供了重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
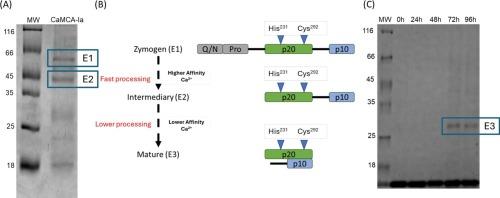
Role of N-terminal of metacaspase of Candida albicans in calcium binding.
Metacaspases are members of the CD clan and share structural similarities with mammalian caspases but possess unique features. This study delves into the Candida albicans metacaspase CaMCA-Ia, a type I metacaspase. CaMCA-Ia demonstrates Ca2+-dependent autoprocessing and presents a hydrophobic N-terminal, which differs from that of type II metacaspases. Truncated CaMCA-Ia (CaMCA-Ia-ΔN86), lacking 86 N-terminal amino acids, undergoes gradual self-processing and intermolecular processing. Calcium addition induces multistep processing, leading to maturation. Like mammalian caspases, CaMCA-Ia-ΔN86 can activate other molecules, indicate intermolecular activation and accelerating maturation. Distinct binding sites for the full-length and truncated forms of CaMCA-Ia in interaction with Ca2+ underscore the N-terminal's role in altering calcium affinity. These findings enhance the understanding of metacaspases' intricate activation and maturation dynamics, offering insights into potential drug targets for pathogenic fungi. The CaMCA mutants D252A, D268A, D299A, and D268/269 A display varied responses to calcium, while the corresponding CaMCA-Ia-ΔN86 mutants exhibit different processing patterns. The D268/299 A mutant showed processing after 48 h of incubation with calcium. Alterations in CaMCA structure and function, such as the deletion of the N-terminus and changes in the aspartates at the calcium-binding site, provide important insights into how CaMCA enzymatic activity and autoprocessing are regulated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


