蜗牛中间寄主栖息地的测绘揭示了地方性环境中血吸虫和非血吸虫吸虫传播的变异性
IF 1.7
Q3 PARASITOLOGY
Current research in parasitology & vector-borne diseases
Pub Date : 2025-01-01
DOI:10.1016/j.crpvbd.2025.100299
引用次数: 0
摘要
血血吸虫是泌尿生殖道血吸虫病的病原,血血吸虫的中间寄主是追踪相关疾病风险传播的关键哨点。除了血单胞菌外,蜗牛还将牛单胞菌传播给牛,并将几种非血吸虫性寄生虫传播给牛和野生动物。确定这些多重寄生虫宿主的传播焦点对于有针对性和有效的“同一健康”干预至关重要。我们调查了坦桑尼亚西北部6个地区86个村庄的467个水体。从2020年11月到2021年8月,在三个调查阶段共收集了43,348只鹦鹉螺。在所有蜗牛中,0.63%的蜗牛散发出血吸虫尾蚴。这一年中,血吸虫流行率显著增加,在旱季(2021年6月至8月)达到高峰。后两期(2021年3 - 8月)采集的25,052只钉螺中,非血吸虫吸虫感染率为4.9%,在所有空间尺度上均高于血吸虫流行率。同时感染不常见,只有0.05%的蜗牛同时携带血吸虫和非血吸虫寄生虫。这些感染模式在村和区各级是一致的。牛用水体的血吸虫流行率高于分离出来供人类使用的水体。令人惊讶的是,在这两种类型的水体中,非血吸虫的流行率是相等的。这表明牛在血吸虫传播中起间接作用,需要将牛和人使用的水体分开,并将旱季的蜗牛控制扩大到牛使用的水体。相比之下,水的持久性和学校附近没有影响蜗牛或寄生虫的存在。有针对性的干预措施应侧重于当地用水动态,并注意牛在血吸虫传播中的潜在间接作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
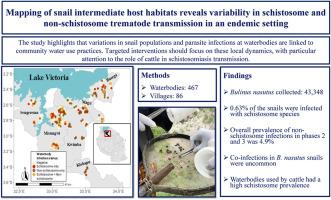
Mapping of snail intermediate host habitats reveals variability in schistosome and non-schistosome trematode transmission in an endemic setting
The intermediate snail host of Schistosoma haematobium, the etiological agent of urogenital schistosomiasis, serves as a critical sentinel for tracking the spread of associated disease risks. In addition to S. haematobium, Bulinus spp. snails also transmit S. bovis to cattle as well as several non-schistosome trematodes to cattle and wildlife. Identifying transmission foci of these multi-parasite hosts is critical for targeted and effective One Health intervention. We investigated 467 waterbodies in 86 villages across six districts in northwestern Tanzania. A total of 43,348 Bulinus nasutus were collected across three survey phases from November 2020 to August 2021. Across all snails, 0.63% were emitting schistosome cercariae. There was a significant increase in schistosome prevalence during the year, with a peak in the dry season (June-August 2021). Furthermore, of the 25,052 snails collected in the latter two phases (March to August 2021), 4.9% were infected with non-schistosome trematodes, exceeding prevalences of schistosomes at all spatial scales. Co-infections were uncommon, with only 0.05% of snails concurrently emitting both schistosome and non-schistosome parasites. These infection patterns were consistent across village and district levels. Waterbodies used by cattle had higher schistosome prevalence than waterbodies isolated for human use. Surprisingly, non-schistosome prevalence was equal in both of these waterbody types. This suggests that cattle have an indirect role in schistosome transmission, requiring the separation of waterbody usage between cattle and humans and extending snail control in dry season to waterbodies used by cattle. By contrast, water permanence and school proximity did not impact snail or parasite presence. Targeted interventions should focus on local water use dynamics, with attention to the potential indirect role of cattle in schistosome transmission.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


