LncRNA Mir155hg通过miR-155/Socs1/NF-κΒ信号轴参与癫痫持续状态大鼠海马损伤
IF 2.9
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景:神经炎症可导致癫痫持续状态(SE)的认知缺陷。lncRNA Mir155hg已被确定为炎症的关键调节因子,但其在SE中的作用尚不清楚。方法应用腺相关病毒(AAV)在SE大鼠模型中敲低smir155hg。采用Morris水迷宫和尼氏染色评估认知功能和神经元损伤。Western blot检测炎症因子(TNF-α、IL-1β)和NF-κB通路活性。通过双荧光素酶测定和共免疫沉淀分析,研究了mir155hg介导的NF-κB调节的机制。结果Mir155hg在体内和体外均与TNF-α和IL-1β的上调呈正相关。Mir155hg的下调降低了海马炎症和NF-κB的激活。在机制上,Mir155hg作为竞争性内源性RNA隔离miR-155,从而抑制Socs1的表达,Socs1是一种靶向NF-κB p65降解的E3连接酶。共免疫沉淀分析证实了NF-κB p65与Socs1之间的相互作用。结论smir155hg通过miR-155/Socs1/NF-κB轴加重se相关神经炎症。靶向这一途径可能减轻se诱导的神经元损伤和认知缺陷。本文章由计算机程序翻译,如有差异,请以英文原文为准。
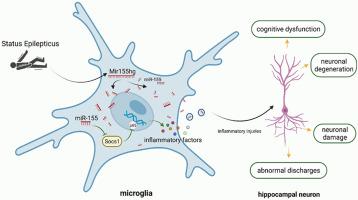
LncRNA Mir155hg contributes to hippocampal injury in status epilepticus rats through miR-155/Socs1/NF-κΒ signaling axis
Background
Neuroinflammation contributes to cognitive deficits in status epilepticus (SE). The lncRNA Mir155hg has been identified as a key regulator of inflammation, but its role in SE remains unclear.
Methods
Mir155hg was knocked down using the adeno-associated virus (AAV) in the rat models of SE. Cognitive function and neuronal damage were assessed using Morris Water Maze and Nissl staining. Inflammatory cytokines (TNF-α, IL-1β) and NF-κB pathway activity were measured by Western blot. Mechanistic insights into Mir155hg-mediated NF-κB regulation were investigated via dual-luciferase assays and co-immunoprecipitation analysis.
Results
Our findings demonstrated that elevated level of Mir155hg was positively correlated with upregulation of TNF-α and IL-1β both in vivo and in vitro. Knockdown of Mir155hg reduced hippocampal inflammation and NF-κB activation. Mechanistically, Mir155hg acted as a competing endogenous RNA to sequester miR-155, thereby suppressing the expression of Socs1, an E3 ligase targeting NF-κB p65 for degradation. Co-immunoprecipitation analysis confirmed the interaction between NF-κB p65 and Socs1.
Conclusions
Mir155hg exacerbates SE-related neuroinflammation via the miR-155/Socs1/NF-κB axis. Targeting this pathway may mitigate SE-induced neuronal injury and cognitive deficits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.20
自引率
2.90%
发文量
104
审稿时长
31 days
期刊介绍:
Journal of Pharmacological Sciences (JPS) is an international open access journal intended for the advancement of pharmacological sciences in the world. The Journal welcomes submissions in all fields of experimental and clinical pharmacology, including neuroscience, and biochemical, cellular, and molecular pharmacology for publication as Reviews, Full Papers or Short Communications. Short Communications are short research article intended to provide novel and exciting pharmacological findings. Manuscripts concerning descriptive case reports, pharmacokinetic and pharmacodynamic studies without pharmacological mechanism and dose-response determinations are not acceptable and will be rejected without peer review. The ethnopharmacological studies are also out of the scope of this journal. Furthermore, JPS does not publish work on the actions of biological extracts unknown chemical composition.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


