新型抗糖尿病药物嘧啶-恶唑复合物的计算设计与合成:动力学与分子相互作用研究
IF 3.1
4区 生物学
Q2 BIOLOGY
引用次数: 0
摘要
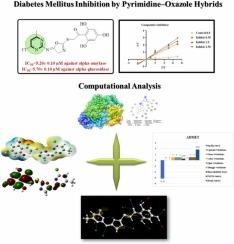
糖尿病(DM)是一种以高血糖为主要特征的复杂的慢性内分泌疾病。糖尿病是由于胰腺不能产生将过量的血糖转化为糖原的胰岛素,或者身体细胞对胰岛素的作用没有反应。糖尿病的主要症状包括视力模糊、排尿过多和溃疡愈合缓慢,但如果不及早诊断和治疗,它可能会导致一些严重的继发性损害,如心血管疾病、糖尿病神经病变、糖尿病肾病和阿尔茨海默病等。目前的研究重点是设计和合成一种新型的以嘧啶为基础的恶唑衍生物(1−10),具有良好的抗糖尿病活性。这些衍生物以试剂级原料4-氯-6-甲基嘧啶-2-胺为原料合成。通过1H-NMR和13C-NMR获得了合成衍生物的结构构象,并通过HREI-MS确定了其分子量。IC50= 10.50 ± 0.20 μM和IC50= 10.80 ± 0.10 μM,与标准阿卡波糖相比,这些化合物对α-淀粉酶和α-葡萄糖苷酶表现出中等至优异的生物学潜力。其中,类似物8对α-淀粉酶的IC50= 5.20 ± 0.10 μM,对α-葡萄糖苷酶的IC50= 5.70 ± 0.10 μM,是该系列中最有效的化合物,对目标酶具有良好的抑制作用。通过分子对接研究新合成的衍生物的生物相互作用,评价其酶抑制效力。此外,还通过分子动力学(MD)模拟、密度泛函理论(DFT)研究来评估它们在动态环境下的结构构象变化、稳定性和反应性。吸收分布、代谢、排泄和毒性(ADMET)分析表明,这些强效化合物无毒理学作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Computationally guided design and synthesis of pyrimidine–oxazole hybrids as novel antidiabetic agents: kinetic and molecular interaction studies
Diabetes mellitus (DM) is one of the complex and chronic endocrine diseases often characterized by high blood glucose level. Diabetes is due to either pancreases not producing insulin which convert excess of blood glucose to glycogen, or the cell of body becoming unresponsive to insulin’s effect. Primary symptom of DM includes blurry vision, excess urination and slow healing sores but if not diagnosed earlier and treated, it is associated with some severe secondary impairment like cardiovascular diseases, diabetic neuropathy, diabetic nephropathy and Alzheimer’s diseases etc. The focus of current research work is to design and synthesized a novel pyrimidine based oxazole derivatives (1−10) having promising anti-diabetic activity. These derivatives were synthesized by using reagent grade starting material i.e. 4-chloro-6-methylpyrimidin-2-amine. Structural conformation of the synthesized derivative was acquired by 1H-NMR and 13C-NMR and their molecular weight were confirmed by HREI-MS. These compound exhibit moderate to excellent biological potential against α-amylase and α-glucosidase in comparison to standard acarbose IC50= 10.50 ± 0.20 μM and IC50= 10.80 ± 0.10 μM. Among these derivatives, analog 8 having IC50= 5.20 ± 0.10 μM against α-amylase and IC50= 5.70 ± 0.10 μM against α-glucosidase emerged as a most potent compound of the series with excellent inhibitory potency of target enzyme. The biological interaction of the newly synthesized derivatives was studied through molecular docking to assess their enzyme inhibition potency. Furthermore, the molecular dynamic (MD) simulation, density functional theory (DFT) studies are also performed in order to assess their structural conformational changes, stability and reactivity under dynamic environment. Absorption distribution metabolism excretion and toxicity (ADMET) analysis showed that these potent compounds have no toxicological effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Biology and Chemistry
生物-计算机:跨学科应用
CiteScore
6.10
自引率
3.20%
发文量
142
审稿时长
24 days
期刊介绍:
Computational Biology and Chemistry publishes original research papers and review articles in all areas of computational life sciences. High quality research contributions with a major computational component in the areas of nucleic acid and protein sequence research, molecular evolution, molecular genetics (functional genomics and proteomics), theory and practice of either biology-specific or chemical-biology-specific modeling, and structural biology of nucleic acids and proteins are particularly welcome. Exceptionally high quality research work in bioinformatics, systems biology, ecology, computational pharmacology, metabolism, biomedical engineering, epidemiology, and statistical genetics will also be considered.
Given their inherent uncertainty, protein modeling and molecular docking studies should be thoroughly validated. In the absence of experimental results for validation, the use of molecular dynamics simulations along with detailed free energy calculations, for example, should be used as complementary techniques to support the major conclusions. Submissions of premature modeling exercises without additional biological insights will not be considered.
Review articles will generally be commissioned by the editors and should not be submitted to the journal without explicit invitation. However prospective authors are welcome to send a brief (one to three pages) synopsis, which will be evaluated by the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


