目前关于斯蒂尔氏病生物疗法转换的证据:系统文献综述
IF 4.4
2区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
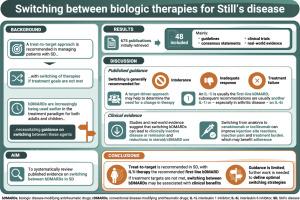
目的:根据从治疗到靶点的方法,越来越多的人使用生物疾病修饰抗风湿药物(bDMARDs)进行治疗,这些药物之间的转换是这一策略的组成部分。然而,指导临床医生在SD中切换生物制剂的指导是有限的。在这里,我们回顾了有关SD中bdmard之间切换的文献证据。方法对2014年以来发表的SD生物治疗相关文献进行系统回顾。排除5岁以下患者的病例报告和研究以及非英语出版物。由此产生的出版物进行了审查,以寻找在bdmard之间转换治疗的证据。结果共纳入48篇出版物,包括7篇治疗指南、共识声明或最佳实践建议,以及41篇临床试验、观察性研究、注册研究或病例系列出版物。白细胞介素(IL)-1抑制剂通常是推荐的一线生物制剂。在缺乏耐受性、反应不足或治疗失败的情况下,建议改用另一种IL-1抑制剂或IL-6抑制剂。肿瘤坏死因子抑制剂和Janus激酶抑制剂被建议用于后期的治疗。临床出版物报道了在bdmard之间切换的益处,包括获得临床非活动性疾病或缓解,类固醇节约效应和减少治疗负担。结论对于未达到治疗目标的SD患者,在bdmard之间切换可能是一种与临床获益相关的有用策略。然而,需要专门设计的对照临床试验(如果没有对照试验,则需要观察性研究)来确定最佳的转换策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Current evidence on switching between biologic therapies for Still’s disease: A systematic literature review
Objective
Still's disease (SD) is increasingly being treated with biologic disease-modifying antirheumatic drugs (bDMARDs) according to a treat-to-target approach, with switching between these agents an integral part of this strategy. However, guidance for the clinician on switching between biologic agents in SD is limited. Here we review the evidence in the literature relating to switching between bDMARDs in SD.
Methods
A systematic review was performed for articles published since 2014 which related to the biologic treatment of SD. Case reports and studies with under 5 patients and non-English language publications were excluded. The resulting publications were reviewed for evidence on switching therapies between bDMARDs.
Results
A total of 48 publications included for review included seven treatment guidelines, consensus statements, or best practice recommendations, and 41 publications of clinical trials, observational studies, registry studies, or case series. Interleukin (IL)-1 inhibitors are usually the recommended first-line biologics. Switching to another IL-1 inhibitor or an IL-6 inhibitor was recommended in cases of lack of tolerability, inadequate response or treatment failure. Tumour necrosis factor inhibitors and Janus kinase inhibitors were suggested for later lines of therapy. Clinical publications reported benefits of switching between bDMARDs in terms of attaining clinically inactive disease or remission, steroid-sparing effects, and reductions in treatment burden.
Conclusion
In patients with SD who are not meeting treatment targets, switching between bDMARDs can be a useful strategy associated with clinical benefits. However, specifically designed, controlled clinical trials (or in their absence, observational studies) are needed to determine optimal switching strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.20
自引率
4.00%
发文量
176
审稿时长
46 days
期刊介绍:
Seminars in Arthritis and Rheumatism provides access to the highest-quality clinical, therapeutic and translational research about arthritis, rheumatology and musculoskeletal disorders that affect the joints and connective tissue. Each bimonthly issue includes articles giving you the latest diagnostic criteria, consensus statements, systematic reviews and meta-analyses as well as clinical and translational research studies. Read this journal for the latest groundbreaking research and to gain insights from scientists and clinicians on the management and treatment of musculoskeletal and autoimmune rheumatologic diseases. The journal is of interest to rheumatologists, orthopedic surgeons, internal medicine physicians, immunologists and specialists in bone and mineral metabolism.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


