YBX1的o - glcn酰化在肝细胞癌中驱动糖酵解-组蛋白乳酸化反馈回路
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
代谢重编程是肿瘤发生和进展的一个标志,葡萄糖代谢的改变,通常被称为Warburg效应,起着核心作用。这种转变允许肿瘤细胞快速获取能量并产生必需的代谢中间体,从而支持增强生长。尽管其意义重大,但肿瘤细胞上调糖酵解的机制仍未充分了解。在这项研究中,我们报道了YBX1在肝细胞癌(HCC)中高表达,并且与糖酵解密切相关。我们发现YBX1在苏氨酸57 (T57)处被O-linked n -乙酰氨基葡萄糖(O-GlcNAc)修饰,从而稳定了该蛋白并增加了其表达。这种修饰还促进YBX1 102丝氨酸的磷酸化,促进其核易位。因此,这一过程增强了糖酵解相关基因的转录并刺激了乳酸的产生。此外,YBX1激活P300的转录,进而驱动组蛋白的乳酸化,尤其是H3K18la。CUT&;Tag分析显示H3K18的乳酸化正调控YBX1基因的转录。我们的研究结果建立了一个涉及YBX1、糖酵解和H3K18乳酸化的正反馈循环,加速了HCC的进展。破坏这种反馈循环可能为HCC提供一种新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
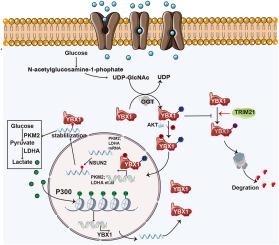
O-GlcNAcylation of YBX1 drives a glycolysis-histone lactylation feedback loop in hepatocellular carcinoma
Metabolic reprogramming is a hallmark of tumorigenesis and progression, with alterations in glucose metabolism, often referred to as the Warburg effect, playing a central role. This shift allows tumor cells to rapidly acquire energy and generate essential metabolic intermediates, thereby supporting enhanced growth. Despite its significance, the mechanisms by which tumor cells upregulate glycolysis remain inadequately understood. In this study, we report that YBX1 is highly expressed in hepatocellular carcinoma (HCC) and is closely associated with glycolysis. We show that YBX1 is modified by O-linked N-acetylglucosamine (O-GlcNAc) at threonine 57 (T57), which stabilizes the protein and increases its expression. This modification also promotes the phosphorylation of YBX1 at serine 102, facilitating its nuclear translocation. Consequently, this process enhances the transcription of glycolysis-related genes and stimulates lactate production. Moreover, YBX1 activates the transcription of P300, which in turn drives the lactylation of histones, particularly H3K18la. Cleavage Under Targets and Tagmentation (CUT&Tag) analysis reveals that H3K18 lactylation positively regulates YBX1 gene transcription. Our findings establish a positive feedback loop involving YBX1, glycolysis, and H3K18 lactylation that accelerates HCC progression. Disrupting this feedback loop may provide a novel therapeutic strategy for HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


