SIX1通过ZEB1/IL6/STAT3信号轴传递乳腺癌进展信号。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
上皮间充质转化(Epithelial-mesenchymal transition, EMT)和乳腺癌干细胞(breast cancer stem cells, BCSCs)是乳腺癌机制研究的关键。研究表明,Sine oculis homobox同源物1 (SIX1)协调乳腺癌EMT和BCSCs,同时激活信号换能器和转录激活器3 (STAT3)信号通路。然而,SIX1调节STAT3的机制及其通过STAT3信号传导调节EMT和BCSCs的潜力仍未被探索。在这里,我们对人乳腺癌标本进行了细胞、动物、类器官模型以及整合的单细胞转录组学和st序列分析。结果表明,SIX1可增强锌指E-box binding homeobox 1 (ZEB1)的表达和翻译,ZEB1与白细胞介素-6 (il -6)启动子(1138bp-1148bp)结合,刺激其转录、翻译和分泌。随后,IL6可以激活细胞自身的STAT3信号通路,促进STAT3的磷酸化,促进下游信号c-Myc和Cyclin D1的转导,促进ALDH1A1、OCT4、SOX2等干细胞相关转录因子的表达,从而促进EMT和干性。此外,本研究发现了一种新的细胞相互作用模型,即上述分泌的IL6可以旁分泌的方式促进周围SIX1低表达细胞STAT3信号通路的激活、EMT和干性转化。我们的数据支持SIX1/ZEB1/IL6轴激活乳腺细胞自身及周围SIX1低表达细胞的STAT3信号通路,从而促进EMT和干性转化,激活整个乳腺癌的恶性进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
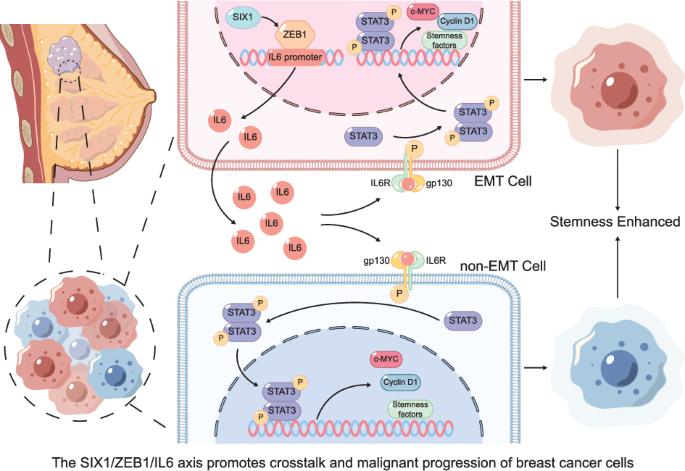
SIX1 transmits signals for breast cancer progression via the ZEB1/IL6/STAT3 signaling axis
Epithelial-mesenchymal transition (EMT) and breast cancer stem cells (BCSCs) are pivotal in breast cancer mechanism research. It was demonstrated that Sine oculis homeobox homolog 1 (SIX1) orchestrates breast cancer EMT and BCSCs, concurrently activating the Signal transducer and activator of transcription 3 (STAT3) signaling pathway. Yet, the mechanism by which SIX1 modulates STAT3 and its potential to regulate EMT and BCSCs through STAT3 signaling remain unexplored. Here, cellular, animal, organoid models, and integrated single-cell transcriptomic and ST-seq of human breast cancer specimens were conducted. The results revealed that SIX1 can enhance Zinc finger E-box binding homeobox 1 (ZEB1) expression and translation, which in turn binds to the Interleukin-6 (IL6) promoter (1138bp–1148bp) to stimulate its transcription, translation, and secretion. Subsequently, IL6 can activate the cell’s own STAT3 signaling pathway, promote the phosphorylation of STAT3, promote the downstream signal c-Myc and Cyclin D1 transduction, and promote the expression of stem cell-related transcription factors such as ALDH1A1, OCT4, and SOX2, thereby promoting EMT and stemness. In addition, this study found a new cell interaction model, in which the above-mentioned secreted IL6 can promote the activation of STAT3 signaling pathway, EMT and stemness transformation in the surrounding cells with low expression of SIX1 in a paracrine manner. Our data favored that SIX1/ZEB1/IL6 axis activated the STAT3 signaling pathway of the breast cells themselves and surrounding cells with low SIX1 expression, thus promoting EMT and stemness transformation, activating the malignant progression of the whole breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


