液-液相分离GPS2-LATS1通过重编程脂质代谢促进结直肠癌进展。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
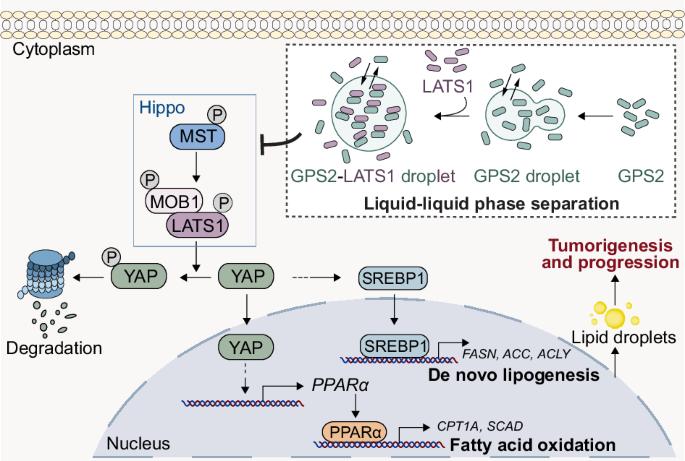
异常脂质代谢是结直肠癌(CRC)的一个标志,但其潜在的调节机制仍不完全清楚。在这里,我们发现G蛋白途径抑制因子2 (GPS2)是一个关键的致癌驱动因素,它协调脂质代谢重编程,促进结直肠癌的进展。临床上,GPS2在结直肠癌中过表达,并与侵袭性表型相关。在功能上,GPS2缺失抑制体外和体内肿瘤生长,而其过表达则加速恶性肿瘤的发生。在机制上,GPS2通过促进甾醇调节元件结合蛋白1 (SREBP1)的核易位激活脂质合成,并通过增加过氧化物酶体增殖因子激活受体α (PPARα)的转录促进脂肪酸氧化,双重调节脂质代谢。至关重要的是,我们发现GPS2在CRC细胞中经历了液-液相分离(LLPS),在那里它形成了促进致癌信号传导的生物分子凝聚物。phase-separated GPS2通过其coil -coil结构域,直接与Hippo通路核心激酶LATS1的c -末端激酶结构域相互作用,诱导LATS1的LLPS并抑制其活性。这种失活释放YAP,进而放大SREBP1/ ppar α驱动的脂质代谢。救援实验证实,在GPS2缺失的情况下,YAP重建可以恢复SREBP1的核易位和PPARα转录,从而确定LATS1-YAP轴是GPS2介导的脂质代谢编程的中心效应体。我们的研究描述了一种新的相分离依赖机制,GPS2在空间上重组LATS1-YAP信号以重编程脂质代谢并促进CRC进展,提示了CRC代谢干预的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Liquid-liquid phase separation of GPS2-LATS1 promotes colorectal cancer progression by reprogramming lipid metabolism
Aberrant lipid metabolism is a hallmark of colorectal cancer (CRC), yet the underlying regulatory mechanisms remain incompletely understood. Here, we identified G protein pathway suppressor 2 (GPS2) as a pivotal oncogenic driver that orchestrates lipid metabolic reprogramming to fuel CRC progression. Clinically, GPS2 is overexpressed in CRC and is correlated with aggressive phenotypes. Functionally, GPS2 depletion inhibits tumor growth in vitro and in vivo, whereas its overexpression accelerates malignancy. Mechanistically, GPS2 dual-regulates lipid metabolism by facilitating the nuclear translocation of sterol regulatory element-binding protein 1 (SREBP1) to activate lipid synthesis and by increasing peroxisome proliferator-activated receptor α (PPARα) transcription to promote fatty acid oxidation. Crucially, we revealed that GPS2 undergoes liquid-liquid phase separation (LLPS) in CRC cells, where it forms biomolecular condensates that promote oncogenic signaling. Through its coiled-coil domain, phase-separated GPS2 directly interacts with the C-terminal kinase domain of large tumor suppressor 1 (LATS1), a core kinase of the Hippo pathway, inducing LLPS of LATS1 and suppressing its activity. This inactivation releases YAP, which in turn amplifies SREBP1/PPARα-driven lipid metabolism. Rescue experiments confirmed that YAP reconstitution restores SREBP1 nuclear translocation and PPARα transcription upon GPS2 loss, establishing the LATS1-YAP axis as the central effector of GPS2-mediated lipid metabolic programming. Our study delineates a novel phase separation-dependent mechanism whereby GPS2 spatially reorganizes LATS1-YAP signaling to reprogram lipid metabolism and promote CRC progression, suggesting potential therapeutic targets for metabolic intervention in CRC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


