新辅助免疫检查点抑制提高对肝黑色素瘤转移的保护。
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
背景:皮肤黑色素瘤(CM)肝转移与免疫检查点抑制(ICI)应答降低相关。在这里,我们研究了新辅助ICI是否可以防止肝转移以防止治疗耐药的发展。方法:通过皮内注射WT31或B16F10 luc2黑色素瘤细胞,建立II期CM模型。联合ICI(抗pd -1/抗ctla -4)应用于小鼠肝黑色素瘤转移模型,比较新辅助或辅助方案。通过scRNA-Seq、流式细胞术、免疫荧光、原位杂交和多种细胞因子检测,比较分析肝脏和CMs中免疫细胞的组成和应答。结果:与辅助治疗相比,新辅助ICI对肝转移的保护作用增强。这种优越的反应与cm、外周血和肝脏中T细胞的扩增有关。通过scRNA-Seq和免疫荧光检测,新辅助ICI后小鼠CMs和肝脏T细胞中th1相关标记物表达增加,th2相关标记物表达下调。肝脏细胞因子分析也显示th2相关的IL-4和IL-15水平较低。结论:我们的数据表明,新辅助ICI对肝黑色素瘤转移提供了卓越的保护,并向抗肿瘤TH1免疫反应转变。因此,新辅助ICI是CM预防器官特异性治疗耐药机制发展的有希望的治疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
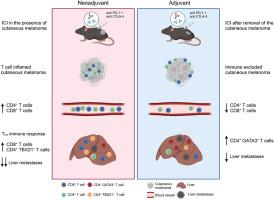
Neoadjuvant immune checkpoint inhibition improves protection against hepatic melanoma metastasis
Background
Liver metastasis of cutaneous melanoma (CM) correlates with a decreased response to immune checkpoint inhibition (ICI). Here, we investigated whether neoadjuvant ICI protects against liver metastasis to prevent the development of therapy resistances.
Methods
A stage II CM was modeled by intracutaneous injections of WT31 or B16F10 luc2 melanoma cells. Combined ICI (anti-PD-1/anti-CTLA-4) was applied in murine models of hepatic melanoma metastasis comparing neoadjuvant or adjuvant regimens. Immune cell composition and responses in the liver and CMs were comparatively analyzed by scRNA-Seq, flow cytometry, immunofluorescence, in situ hybridization and multiplex cytokine assays.
Results
Neoadjuvant ICI resulted in improved protection against liver metastasis in comparison to adjuvant therapy. This superior response was associated with an expansion of T cells in CMs, the peripheral blood and the liver. An increased expression of TH1-associated markers and a downregulation of TH2-associated markers were detected in T cells from CMs and livers of mice by scRNA-Seq and immunofluorescence after neoadjuvant ICI. Analysis of hepatic cytokines also revealed lower levels of TH2-associated IL-4 and of IL-15.
Conclusion
Our data demonstrate that neoadjuvant ICI provides superior protection against hepatic melanoma metastasis with a shift towards an anti-tumor TH1 immune response. Therefore, neoadjuvant ICI is a promising therapeutic option for CM to prevent the development of organ-specific therapy resistance mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


