基于Li簇的气体传感器的计算分析
IF 3
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
锂簇由于其可调谐的电子特性和高反应性而成为气敏应用的有希望的候选者。在这项研究中,我们系统地研究了CO、CO2、CS2、SO2、NO、NO2、H2S和NH3在Li簇上的吸附行为。结构和电子分析表明,增加团簇尺寸通过更强的金属键增强稳定性,而奇数团簇表现出自旋极化,影响其化学活性。吸附能计算证实,CO, CO2, H2S和NH3在最佳能量范围(0.2-0.7 eV)内相互作用,确保可逆和选择性传感。灵敏度分析表明,NH3和H2S是最易探测的气体,因为它们具有很强的电荷再分配效应。偶极矩的变化与吸附强度相关,进一步加强了静电相互作用在气体检测中的作用。态密度(DOS)和还原密度梯度(RDG)分析强调了气体吸附过程中显著的电子变化,证实了电荷转移和相互作用机制。Li簇在CO, CO2, H2S和NH3的选择性,吸附能量和恢复时间方面表现出最佳性能,使其成为气敏应用中非常有前途的候选者。然而,在潮湿或富氧环境中,H2O、O2和O2的竞争性吸附对实际应用提出了挑战。这些发现为Li簇的气体传感能力提供了关键的见解,并为其在实际传感器开发中的优化铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
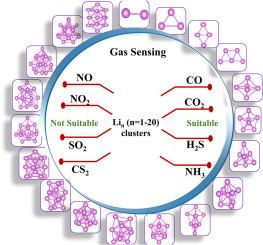
Computational insights into Li cluster–based gas sensors
Lithium clusters have emerged as promising candidates for gas sensing applications due to their tunable electronic properties and high reactivity. In this study, we systematically investigate the adsorption behaviour of CO, CO2, CS2, SO2, NO, NO2 H2S, and NH3 on Li clusters using Density Functional Theory (DFT). Structural and electronic analysis reveals that increasing cluster size enhances stability through stronger metallic bonding, while odd–numbered clusters exhibit spin polarization, influencing their chemical activity. Adsorption energy calculations confirm that CO, CO2, H2S, and NH3 interact within the optimal energy range (0.2–0.7 eV), ensuring reversible and selective sensing. Sensitivity analysis identifies NH3 and H2S as the most detectable gases due to their strong charge redistribution effects. Dipole moment variations correlate with adsorption strength, further reinforcing the role of electrostatic interactions in gas detection. Density of States (DOS) and Reduced Density Gradient (RDG) analyses highlight significant electronic modifications upon gas adsorption, confirming charge transfer and interaction mechanisms. Li clusters demonstrate best performance in selectivity, adsorption energy, and recovery time for CO, CO2, H2S, and NH3, making them highly promising candidates for gas sensing applications. However, competitive adsorption from H2O, O2, and O2 in humid or oxygen–rich environments present challenges for real–world applications. These findings provide key insights into the gas sensing capabilities of Li clusters and pave the way for their optimization in practical sensor development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


