亚慢性暴露于CeCl3可通过抑制Keap1/Nrf2/GPX4通路促进巨噬细胞铁凋亡和动脉粥样硬化斑块形成
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
氯化铈(Cerium chloride, CeCl3)是稀土开采过程中普遍存在的副产品,在矿区的植物、动物、水资源和空气等生物群和环境中积累,对当地居民的健康构成潜在威胁。动脉粥样硬化(AS)是心血管疾病的病理基础。然而,亚慢性CeCl3暴露与AS进展之间的关系,以及CeCl3调节AS的潜在调节机制仍不清楚。在这项研究中,我们首次证实了CeCl3显著加剧了高脂肪饮食(HFD)喂养的ApoE−/−小鼠AS斑块的形成。此外,CeCl3提高了血清甘油三酯(TG)、胆固醇(CHO)和低密度脂蛋白(LDL)水平。进一步研究表明,CeCl3显著抑制铁凋亡关键调控因子GPX4、FTH1以及抗氧化酶谷胱甘肽过氧化物酶4 (GPX4)、超氧化物歧化酶(SOD)的表达,上调活性氧(ROS)、丙二醛(MDA)、亚铁(Fe2+)等脂质过氧化标志物水平。此外,CeCl3显著抑制Nrf2、NQO1和HO-1的表达,阻碍Nrf2核易位。在机制上,CeCl3显著促进Keap1/Nrf2复合物的形成,导致泛素介导的Nrf2降解。NK-252药理激活Nrf2可显著降低cecl3诱导的GPX4和FTH1表达抑制,逆转其促动脉粥样硬化作用。双荧光素酶报告基因试验证实Nrf2是GPX4的转录调节因子。综上所述,我们的研究表明CeCl3通过Keap1/Nrf2/GPX4信号通路加剧了hfd诱导的AS斑块形成,促进巨噬细胞铁凋亡,为预防亚慢性暴露于CeCl3的心血管疾病的策略提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
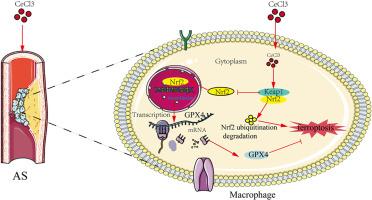
Subchronic exposure to CeCl3 promotes macrophage ferroptosis and atherosclerotic plaque formation by inhibiting the Keap1/Nrf2/GPX4 pathway
Cerium chloride (CeCl3), a prevalent by-product of rare earth mining, accumulates in the biota and environment of mining regions, including plants, animals, water resources, and air, posing potential health risks to local residents. Atherosclerosis (AS) is the pathological basis of cardiovascular disease. However, the relationship between subchronic CeCl3 exposure and AS progression, along with the underlying regulatory mechanisms by which CeCl3 modulates AS, remains unclear. In this study, we first demonstrated confirmed that CeCl3 significantly exacerbated AS plaque formation in ApoE−/− mice fed with high-fat diet (HFD). Moreover, CeCl3 elevated serum levels of triglycerides (TG), cholesterol (CHO), and low-density lipoprotein (LDL). Further studies showed that CeCl3 significantly inhibited the expression of key regulators of ferroptosis, such as GPX4 and FTH1, as well as antioxidant enzyme glutathione peroxidase 4 (GPx4) and superoxide dismutase (SOD), and upregulated the levels of lipid peroxidation markers, including reactive oxygen species (ROS), malondialdehyde (MDA), and ferrous iron (Fe2+). Besides, CeCl3 significantly inhibited the expression of Nrf2, NQO1, and HO-1, and impeded Nrf2 nuclear translocation. Mechanistically, CeCl3 significantly promoted the formation of the Keap1/Nrf2 complex, leading to ubiquitin-mediated Nrf2 degradation. Pharmacological activation of Nrf2 by NK-252 significantly reduced CeCl3-induced inhibition of GPX4 and FTH1 expression, and reversed its pro-atherosclerotic effects. Dual-luciferase reporter assays confirmed Nrf2 as a transcriptional regulator of GPX4. Taken together, our study demonstrates that CeCl3 exacerbates HFD-induced AS plaque formation and promotes ferroptosis in macrophages via the Keap1/Nrf2/GPX4 signaling pathway, providing new insights into strategies for preventing cardiovascular diseases with subchronic exposure to CeCl3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


