街头样本中海洛因的二维核磁共振检测与定量
IF 2.2
3区 医学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
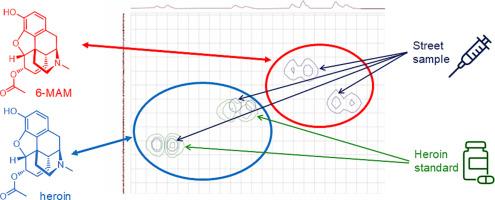
利用二维核磁共振(2D NMR)技术对一种未知街头毒品样品中的化合物进行鉴定和定量。利用2D COSY和HSQC技术,成功定量了海洛因,并通过部分结构解析证实了6-单乙酰吗啡(6-MAM)、噻嗪和咖啡因的存在。这些方法证明了在不依赖参考图书馆数据库的情况下区分结构相似的阿片类药物类似物的能力。虽然气相色谱-质谱(GC-MS)仍然是法医实验室的标准,但它在新生结构分析和检测光谱库中缺失的新兴类似物方面存在局限性。在这项研究中,海洛因和芬太尼在模拟和实际街道样本中被量化,浓度范围为0.97至1.80 mg/mL,使用400 MHz核磁共振仪器,误差在0%至34%之间。台式60 MHz核磁共振系统也检测和定量56毫克/毫升的海洛因在模拟样品24%的误差。鉴于阿片类药物流行和不断发展的药物市场,这些发现支持二维核磁共振波谱在法医药物分析中的补充作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
2D NMR detection and quantification of heroin in a street sample
Two-dimensional nuclear magnetic resonance (2D NMR) spectroscopy was evaluated for the identification and quantification of compounds in an unknown street drug sample. Using 2D COSY and HSQC techniques, heroin was successfully quantified, and the presence of 6-monoacetylmorphine (6-MAM), xylazine, and caffeine was confirmed through partial structural elucidation. These methods demonstrated the ability to differentiate structurally similar opioid analogues without reliance on reference library databases. While gas chromatography–mass spectrometry (GC–MS) remains the standard in forensic laboratories, it has limitations in de novo structural analysis and in detecting emerging analogues absent from spectral libraries. In this study, heroin and fentanyl were quantified in both simulated and actual street samples at concentrations ranging from 0.97 to 1.80 mg/mL, with errors between 0 % and 34 % using a 400 MHz NMR instrument. A benchtop 60 MHz NMR system also detected and quantified 56 mg/mL of heroin with a 24 % error in a simulated sample. These findings support the complementary role of 2D NMR spectroscopy in forensic drug analysis in light of the opioid epidemic and the evolving drug market.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Forensic Chemistry
CHEMISTRY, ANALYTICAL-
CiteScore
5.70
自引率
14.80%
发文量
65
审稿时长
46 days
期刊介绍:
Forensic Chemistry publishes high quality manuscripts focusing on the theory, research and application of any chemical science to forensic analysis. The scope of the journal includes fundamental advancements that result in a better understanding of the evidentiary significance derived from the physical and chemical analysis of materials. The scope of Forensic Chemistry will also include the application and or development of any molecular and atomic spectrochemical technique, electrochemical techniques, sensors, surface characterization techniques, mass spectrometry, nuclear magnetic resonance, chemometrics and statistics, and separation sciences (e.g. chromatography) that provide insight into the forensic analysis of materials. Evidential topics of interest to the journal include, but are not limited to, fingerprint analysis, drug analysis, ignitable liquid residue analysis, explosives detection and analysis, the characterization and comparison of trace evidence (glass, fibers, paints and polymers, tapes, soils and other materials), ink and paper analysis, gunshot residue analysis, synthetic pathways for drugs, toxicology and the analysis and chemistry associated with the components of fingermarks. The journal is particularly interested in receiving manuscripts that report advances in the forensic interpretation of chemical evidence. Technology Readiness Level: When submitting an article to Forensic Chemistry, all authors will be asked to self-assign a Technology Readiness Level (TRL) to their article. The purpose of the TRL system is to help readers understand the level of maturity of an idea or method, to help track the evolution of readiness of a given technique or method, and to help filter published articles by the expected ease of implementation in an operation setting within a crime lab.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


