大麻二酚是否能减少大麻对精神分裂症的不良影响?一项随机、双盲、交叉试验。
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
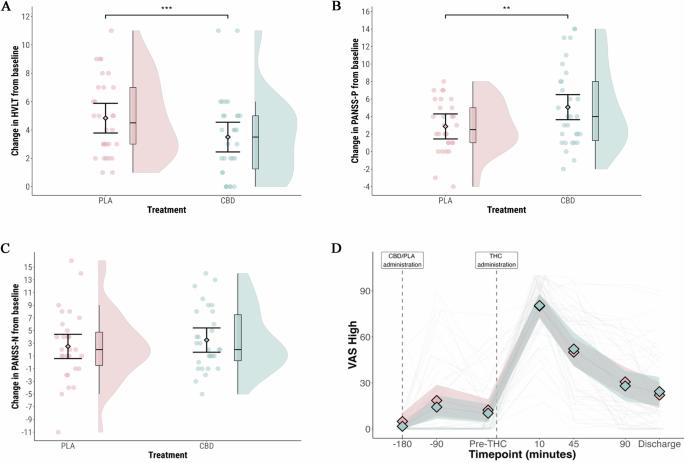
在精神分裂症患者中,使用大麻会加重症状,并可能导致精神病复发。一些针对健康志愿者的实验研究表明,用大麻二酚(CBD)进行预处理可能会减少这些影响,但其他研究则没有。在这里,我们研究了CBD预处理是否能改善精神分裂症患者大麻的急性不良反应。参与者(n = 30)患有精神分裂症或分裂情感性障碍并伴有大麻使用障碍。在一项双盲、随机、安慰剂对照的交叉试验中,参与者在吸入蒸发大麻(含有Δ9-tetrahydrocannabinol (THC) 20-60毫克)前3小时口服CBD 1000毫克或安慰剂。主要结果是用霍普金斯语言学习测试(修订版)测量延迟语言回忆。我们还用阳性和阴性症状量表(PANSS) -阳性亚量表测量精神病症状。服用大麻后,CBD预处理后的延迟言语回忆为3.5个词(95%可信区间[CI]: 2.5-4.5),而安慰剂预处理后的延迟言语回忆为4.8个词(95% CI: 3.9 - 5.8)(平均差异[MD] = -1.3 [95% CI: -2.0至-0.6];p = 0.001)。CBD预处理后,吸入大麻与PANSS-P评分增加相关,为5.0 (95% CI: 3.6至6.5),而安慰剂预处理后为2.9 (95% CI: 1.5至4.3)(MD = 2.2 [95% CI: 0.6至3.7];p = 0.01)。给药CBD对四氢大麻酚及其活性代谢物11-羟基四氢大麻酚的血浆浓度没有显著影响。在精神分裂症和合并症大麻使用障碍患者中,CBD预处理并没有减轻大麻对记忆障碍或精神病症状的急性影响,反而似乎加剧了它们。该研究已在Clinicaltrials.gov注册(NCT04605393)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Does cannabidiol reduce the adverse effects of cannabis in schizophrenia? A randomised, double-blind, cross-over trial
In patients with schizophrenia, cannabis use exacerbates symptoms and can lead to a relapse of psychosis. Some experimental studies in healthy volunteers suggest that pre-treatment with cannabidiol (CBD) may reduce these effects, but others do not. Here, we investigated whether pre-treatment with CBD ameliorates the acute adverse effects of cannabis in patients with schizophrenia. Participants (n = 30) had schizophrenia or schizoaffective disorder plus a comorbid cannabis use disorder. In a double-blind, randomised, placebo-controlled, crossover trial, participants received oral CBD 1000 mg or placebo three hours before inhaling vaporised cannabis (containing Δ9-tetrahydrocannabinol (THC) 20–60 mg). The primary outcome was delayed verbal recall measured with the Hopkins Verbal Learning Test-Revised. We also measured psychotic symptoms with the Positive and Negative Syndrome Scale (PANSS) – positive subscale. Delayed verbal recall after cannabis administration was 3.5 words (95% confidence interval [CI]: 2.5–4.5) following pre-treatment with CBD, compared to 4.8 words (95% CI: 3.9 to 5.8) following pre-treatment with placebo (mean difference [MD] = −1.3 [95% CI: −2.0 to −0.6]; p = 0.001). After CBD pre-treatment, inhalation of cannabis was associated with an increase in PANSS-P score of 5.0 (95% CI: 3.6 to 6.5), compared to 2.9 (95% CI: 1.5 to 4.3) following pre-treatment with placebo (MD = 2.2 [95% CI: 0.6 to 3.7]; p = 0.01). Administration of CBD did not have a significant effect on plasma concentration of THC or its active metabolite, 11-hydroxy-THC. In patients with schizophrenia and a comorbid cannabis use disorder, pre-treatment with CBD did not attenuate the acute effects of cannabis on memory impairment or psychotic symptoms, but appeared to exacerbate them. The study was registered on Clinicaltrials.gov (NCT04605393).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


