PMCA2过表达对Ca2+信号的衰减影响小胶质细胞对病理事件的反应。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
小胶质细胞对脑疾病和损伤的病理过程有重要影响。细胞内Ca2+动力学作为中央整合器,将小胶质细胞的感知能力与其反应联系起来。我们产生了一个小胶质细胞过表达质膜Ca2+- atp酶(PMCA)2的小鼠系,PMCA 2是细胞质Ca2+稳态的中央调节剂。这种操作显著减弱了atp在体外引起的Ca2+信号和体内自发的Ca2+瞬态。值得注意的是,与星形胶质细胞相比,PMCA2在小胶质细胞/巨噬细胞中的过表达并不影响动物的行为和存活。然而,在病理背景下,它对小胶质细胞的反应性有深远的影响,包括减少脂多糖刺激后的炎症反应和减少急性损伤部位的小胶质细胞增殖。在阿尔茨海默病模型中,PMCA2过表达减弱了疾病相关的小胶质细胞特征,减少了淀粉样斑块负担和斑块相关的神经性营养不良。这些发现强调了Ca2+介导的信号对于调节小胶质细胞对病理事件的反应的重要性。PMCA2过表达减弱小胶质细胞Ca2+信号是在脑炎症或变性中促进有益小胶质细胞表型的潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
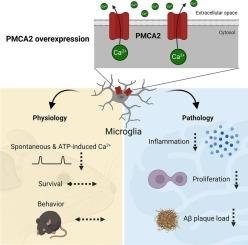
Attenuation of Ca2+ signaling by overexpression of PMCA2 affects the microglial response to pathological events
Microglia strongly impact the pathologic course of brain diseases and injuries. Intracellular Ca2+ dynamics serve as central integrators, connecting microglial sensing capacity to their responses. We generated a mouse line with microglial overexpression of plasma membrane Ca2+-ATPase (PMCA)2, a central regulator of cytoplasmic Ca2+ homeostasis. This manipulation significantly attenuated ATP-evoked Ca2+ signals in vitro and spontaneous Ca2+ transients in vivo. Notably, in contrast to astrocytes, PMCA2 overexpression in microglia/macrophages did not affect animal behavior and survival. It had, however, a profound impact on microglial reactivity in pathological contexts, including reduced inflammatory responses following lipopolysaccharide challenge and diminished microglial proliferation at sites of acute injury. In an Alzheimer’s disease model, PMCA2 overexpression attenuated the disease-associated microglial signature, reducing amyloid plaque burden and plaque-associated neuritic dystrophy. These findings highlight the importance of Ca2+-mediated signaling for modulating the microglial response to pathologic events. Attenuating microglial Ca2+ signaling by PMCA2 overexpression is a potential strategy to promote beneficial microglial phenotypes in brain inflammation or degeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


