NVP-BEZ235通过靶向tau病变增强自噬并改善认知缺陷
IF 3.7
4区 医学
Q2 PATHOLOGY
引用次数: 0
摘要
tau病是一类神经退行性疾病,其特征是过度磷酸化tau蛋白(p-tau)的异常积累和神经原纤维缠结的形成。自噬是一种基本的细胞降解途径,通过促进错误折叠和聚集蛋白的清除,在维持蛋白质稳态中起着关键作用。然而,在tau病变中,自噬过程经常受损,导致p-tau的病理积累。NVP-BEZ235是哺乳动物雷帕霉素靶点(mTOR)和PI3K的双重抑制剂,此前已在实体瘤和淋巴瘤的I期临床试验中进行了评估。在这项研究中,我们在体外和体内研究了NVP-BEZ235在牛头病模型中的治疗潜力。在稳定表达人类p301l突变型tau (SH-Tau)的SH-SY5Y细胞中,NVP-BEZ235处理诱导LC3B-II的时间依赖性增加和p62水平的降低,与增强的自噬活性一致。自噬通量分析进一步证实mTOR通路抑制对自噬的促进作用。NVP-BEZ235显著降低tau蛋白多个残基的磷酸化,包括Ser262、Ser396、Ser404和Thr231,而不引起细胞毒性作用。在转基因小鼠tau病模型(P301S)中,长期使用NVP-BEZ235 (20 mg/kg/天,持续两个月)导致大脑中ripa可溶性和-不溶性p-tau物质的显著减少。通过Morris水迷宫和新物体识别测试评估,实验组小鼠的空间学习和记忆显著改善。此外,NVP-BEZ235降低了神经炎症标志物和促炎细胞因子(TNF-α, IL-1β, IL-6)水平,同时也增强了脑组织中的自噬标志物。血液学分析和器官组织学未发现系统性毒性的迹象。总的来说,这些发现表明NVP-BEZ235通过抑制mTOR增强自噬,从而促进tau清除,从而减轻tau病模型中的认知缺陷和神经炎症。该研究支持NVP-BEZ235作为治疗tau相关神经退行性疾病的有希望的候选药物的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
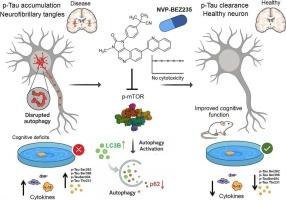
NVP-BEZ235 enhances autophagy and ameliorates cognitive deficits by targeting tauopathies
Tauopathies are a class of neurodegenerative disorders characterized by the abnormal accumulation of hyperphosphorylated tau (p-tau) and the formation of neurofibrillary tangles. Autophagy, a fundamental cellular degradation pathway, plays a pivotal role in maintaining proteostasis by facilitating the clearance of misfolded and aggregated proteins. In tauopathies, however, autophagic processes are often impaired, contributing to the pathological buildup of p-tau. NVP-BEZ235, a dual inhibitor of the mammalian target of rapamycin (mTOR) and PI3K, has previously been evaluated in phase I clinical trials for solid tumors and lymphomas. In this study, we investigated the therapeutic potential of NVP-BEZ235 in tauopathy models, both in vitro and in vivo. In SH-SY5Y cells stably expressing human P301L-mutant tau (SH-Tau), NVP-BEZ235 treatment induced a time-dependent increase in LC3B-II and a decrease in p62 levels, consistent with enhanced autophagic activity. Autophagic flux analysis further confirmed the promotion of autophagy upon mTOR pathway inhibition. NVP-BEZ235 significantly reduced tau phosphorylation at multiple residues, including Ser262, Ser396, Ser404, and Thr231, without eliciting cytotoxic effects. In a transgenic mouse model of tauopathy (P301S), chronic treatment with NVP-BEZ235 (20 mg/kg/day for two months) resulted in a marked reduction of both RIPA-soluble and -insoluble p-tau species in the brain. Spatial learning and memory, assessed through Morris water maze and novel object recognition tests, were significantly improved in treated mice. Furthermore, NVP-BEZ235 administration reduced neuroinflammatory markers and pro-inflammatory cytokine levels (TNF-α, IL-1β, IL-6), while also enhancing autophagic markers in brain tissue. Hematological analysis and organ histology revealed no signs of systemic toxicity. Collectively, these findings demonstrate that NVP-BEZ235 facilitates tau clearance by enhancing autophagy through mTOR inhibition, thereby mitigating cognitive deficits and neuroinflammation in tauopathy models. This study supports the therapeutic potential of NVP-BEZ235 as a promising candidate for the treatment of tau-related neurodegenerative diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.90
自引率
0.00%
发文量
78
审稿时长
11.5 weeks
期刊介绍:
Under new editorial leadership, Experimental and Molecular Pathology presents original articles on disease processes in relation to structural and biochemical alterations in mammalian tissues and fluids and on the application of newer techniques of molecular biology to problems of pathology in humans and other animals. The journal also publishes selected interpretive synthesis reviews by bench level investigators working at the "cutting edge" of contemporary research in pathology. In addition, special thematic issues present original research reports that unravel some of Nature''s most jealously guarded secrets on the pathologic basis of disease.
Research Areas include: Stem cells; Neoangiogenesis; Molecular diagnostics; Polymerase chain reaction; In situ hybridization; DNA sequencing; Cell receptors; Carcinogenesis; Pathobiology of neoplasia; Complex infectious diseases; Transplantation; Cytokines; Flow cytomeric analysis; Inflammation; Cellular injury; Immunology and hypersensitivity; Athersclerosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


