新型香豆素-恶二唑衍生物α-葡萄糖苷酶和PTP1B抑制剂的设计、合成、动力学分析、分子对接及机理研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
α-葡萄糖苷酶和蛋白酪氨酸磷酸酶1B (PTP1B)是糖尿病治疗的重要靶点,抑制它们的活性可以通过调节胰岛素信号通路,延缓碳水化合物的吸收,增强胰岛素敏感性。本研究以这些酶为目标,制备了新的香豆素-恶二唑衍生物。其中,化合物5j对α-葡萄糖苷酶和PTP1B具有双重抑制活性,IC₅₀值分别为30.57±0.22 μM和7.58±1.96 μM。动力学实验表明其为混合型α-葡萄糖苷酶抑制剂。紫外和红外光谱实验表明,它可以诱导α-葡萄糖苷酶的构象变化,而圆二色光谱实验表明,它可以改变α-葡萄糖苷酶和PTP1B的构象。分子对接结果表明,该化合物可以嵌入到两种酶的活性口袋中。蔗糖负荷试验表明,它能降低体内餐后血糖,对正常细胞具有较低的细胞毒性。综上所述,化合物5j作为一种多靶点抑制剂,在探索和创新新型降糖药物方面具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
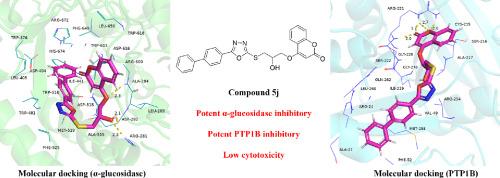
Design, synthesis, kinetic analysis, molecular docking, and mechanistic studies of novel coumarin-oxadiazole derivatives as α-glucosidase and PTP1B inhibitors
α-Glucosidase and protein tyrosine phosphatase 1B (PTP1B) are crucial targets for diabetes treatment, and inhibiting their activity simultaneously can delay the absorption of carbohydrates and enhance insulin sensitivity by modulating the insulin signaling pathway. In this study, novel coumarin-oxadiazole derivatives were prepared by targeting these enzymes. Among them, compound 5j exhibited dual inhibitory activity against α-glucosidase and PTP1B with IC₅₀ values of 30.57 ± 0.22 μM and 7.58 ± 1.96 μM, respectively. Kinetic experiments indicated it was a mixed-type α-glucosidase inhibitor. Ultraviolet and infrared spectroscopy experiments showed it could induce conformational changes in α-glucosidase, while circular dichroism spectroscopy experiments demonstrated the ability to alter the conformations of both α-glucosidase and PTP1B. Molecular docking results revealed that the compound could embed into the active pockets of both enzymes. The sucrose-loading test showed it could reduce post-prandial blood glucose in vivo, and it had low cytotoxicity in normal cells. In conclusion, as a multi-target inhibitor, compound 5j shows great potential in the exploration and innovation of novel anti-hyperglycemic medications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


