细骨草增强了实体瘤免疫治疗的疗效
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
免疫疗法的出现使癌症治疗发生了革命性的变化。然而,免疫治疗耐药性仍然是广泛临床应用的主要障碍。最近的研究强调肠道微生物群通过调节抗肿瘤免疫来增强免疫治疗。在我们的研究中,我们发现,在多个队列中,细叶蝉(A. finegoldii)与优越的免疫治疗疗效相关。随后的体外和体内实验表明,细尾草通过CXCL16-CXCR6轴增强CD8+ T细胞趋化性,增强免疫治疗效果。从机制上说,一种来自金樟的脂蛋白(LIPOAF)通过与toll样受体2结合,激活核因子κB (NF-κB)信号通路,增加CCR7+常规树突状细胞中CXCL16的表达。释放的CXCL16随后促进CXCR6+CD8+ T细胞募集进入肿瘤微环境,有效抑制肿瘤生长。我们的发现提示了一种很有希望的治疗实体瘤的策略,即将细叶假单胞虫与免疫疗法相结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
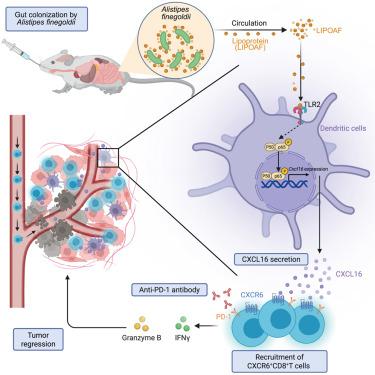
Alistipes finegoldii augments the efficacy of immunotherapy against solid tumors
The emergence of immunotherapy has revolutionized cancer therapy. However, immunotherapeutic resistance remains a major obstacle for broader clinical application. Recent studies highlight that gut microbiota enhances immunotherapy by modulating anti-tumor immunity. In our investigation, we identify that Alistipes finegoldii (A. finegoldii) is associated with superior immunotherapy efficacy across multiple cohorts. Subsequent in vitro and in vivo experiments reveal that A. finegoldii enhances CD8+ T cell chemotaxis via the CXCL16-CXCR6 axis, enhancing immunotherapy efficacy. Mechanistically, a lipoprotein derived from A. finegoldii (LIPOAF) activates the nuclear factor kappa B (NF-κB) signaling pathway to augment CXCL16 expression in CCR7+ conventional dendritic cells through binding with the Toll-like receptor 2. Released CXCL16 subsequently facilitates the recruitment of CXCR6+CD8+ T cells into the tumor microenvironment, effectively curbing tumor growth. Our findings suggest a promising strategy for treating solid tumors by combining A. finegoldii with immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


