NSD2通过整合素/FAK/AKT信号传导促进多囊肾病向小管囊性肾细胞癌的转变。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肾细胞癌是泌尿系统最常见的恶性肿瘤之一。NSD2是一种h3k36特异性二甲基转移酶,据报道参与多种生物过程和人类肿瘤。然而,其在RCC中的作用尚不清楚。在这里,我们发现NSD2在RCC中高表达,这与RCC患者的低生存率相关。NSD2促进myc诱导的多囊肾病向小管囊性肾细胞癌(TCRCC)的转变,TCRCC是一种罕见的RCC亚型,具有独特的临床病理和遗传特征。肾特异性过表达MYC和NSD2 (KMN)的小鼠仅在6周龄时表现出严重的囊肿负担,并在12周龄时发展为TCRCC。机制上,NSD2通过转录上调整合素(Itga4和Itga11)的表达,进一步激活FAK/AKT通路。此外,我们发现NSD2在PEGDA水凝胶的坚硬基质上促进细胞增殖。此外,抑制FAK信号可以缓解KMN小鼠的症状,并在体外显著拯救NSD2过表达引起的细胞增殖增强。总之,我们的研究结果强调了NSD2通过整合素/FAK/AKT通路调控TCRCC肿瘤发生的表观遗传机制。这项研究也可能为开发针对NSD2扩增或高表达的TCRCC患者的靶向治疗铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
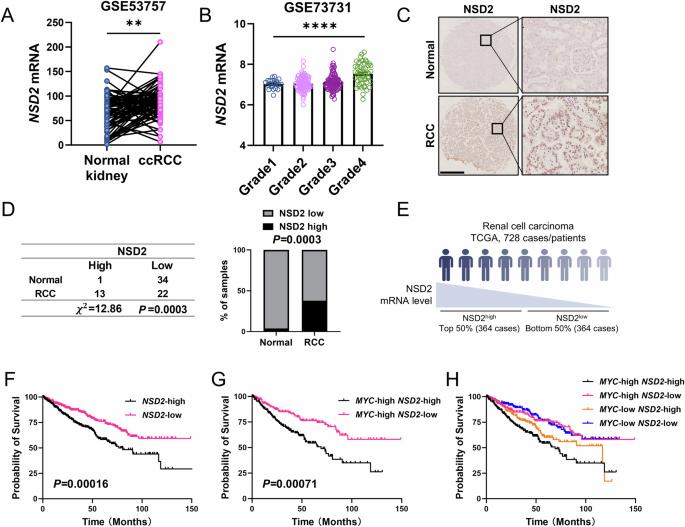
NSD2 promotes cell durotaxis and drives the transition from polycystic kidney disease to tubulocystic renal cell carcinoma through integrin/FAK/AKT signaling
Renal cell carcinoma (RCC) is one of the most common malignancies in the urinary system. NSD2 is an H3K36-specific di-methyltransferase that has been reported to participate in diverse biological processes and human tumors. However, its role in RCC remains unclear. Here, we found that NSD2 is highly expressed in RCC, which is associated with poor survival in RCC patients. NSD2 facilitates the transition from Myc-induced polycystic kidney disease to tubulocystic renal cell carcinoma (TCRCC), which is a rare RCC subtype with distinctive clinicopathologic and genetic characterizations. The mice with kidney-specific overexpression of MYC and NSD2 (KMN) display severe cyst burden at only 6 weeks of age, and develop into TCRCC at 12 weeks of age. Mechanistically, NSD2 transcriptionally upregulates the expressions of integrins (Itga4 and Itga11), to further activate the FAK/AKT pathway. In addition, we found that NSD2 enhances cell proliferation on the stiff matrix of PEGDA hydrogel. Moreover, inhibition of FAK signaling relieves the symptoms of KMN mice, and significantly rescues the enhanced cell proliferation caused by NSD2 overexpression in vitro. Together, our findings highlight an epigenetic mechanism by which NSD2 regulates TCRCC tumorigenesis through the integrin/FAK/AKT pathway. This study may also pave the way for the development of targeted, patient-tailored therapies for TCRCC patients with NSD2 amplification or high expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


