天冬氨酸和聚吡咯功能化多壁碳纳米管对四环素的吸附:动力学、等温线和热力学分析
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了一种由多壁碳纳米管组成的新型吸附剂对四环素的吸附性能,该吸附剂由天冬氨酸和聚吡咯功能化组成。通过批量吸附实验,考察了pH、接触时间、温度和初始浓度对吸附效率的影响。在pH为5,温度为25℃,初始TC浓度为100 mg/L时,最大吸附量为38.08 mg/g。动力学数据最好用拟二阶模型描述,表明是化学吸附,而平衡数据与Langmuir等温线模型吻合较好,证实是单层吸附。热力学参数表明,吸附过程为自发放热过程,ΔG°的取值范围为-11.9 ~ -11.11 kJ/mol, ΔH°= -21.34 kJ/mol。这些发现表明MWCNT-Asp-PPy是一种高效、高容量的TC吸附剂,在水处理中具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
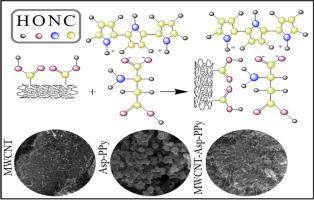
Adsorption of tetracycline using aspartic acid and polypyrrole-functionalized multiwalled carbon nanotubes: Kinetic, isotherm, and thermodynamic analysis
This study investigates the adsorption of tetracycline from aqueous solutions using a novel nanocomposite adsorbent composed of multi-walled carbon nanotubes functionalized with aspartic acid and poly-pyrrole. Batch adsorption experiments were conducted to evaluate the effects of pH, contact time, temperature, and initial concentration on adsorption efficiency. The maximum adsorption capacity was found to be 38.08 mg/g at pH 5, 25 °C, and an initial TC concentration of 100 mg/L. Kinetic data were best described by the pseudo-second-order model, indicating chemisorption, while equilibrium data fit well with the Langmuir isotherm model, confirming monolayer adsorption. Thermodynamic parameters revealed that the adsorption process is spontaneous and exothermic, with ΔG° values ranging from –11.9 to –11.11 kJ/mol and ΔH° = -21.34 kJ/mol. These findings suggest that MWCNT-Asp-PPy is an efficient, high-capacity adsorbent for TC removal, with promising applications in water treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


