棕榈酸盐诱导的脂钙素-前列腺素D2合酶下调伴HepG2细胞肝脂质积累。
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
代谢功能障碍相关脂肪变性肝病(MASLD)与多种代谢功能障碍相关,是全球健康面临的重大挑战。我们之前的体内研究表明,脂钙素前列腺素D2合成酶(L-PGDS)的缺乏会导致脂肪性肝病的发生,当C57BL/6小鼠保持高脂肪饮食时,L-PGDS的表达显著降低。简而言之,L-PGDS属于花生四烯酸途径,可将前列腺素H2酶异构化为前列腺素D2,并通过DP1和DP2两种受体发挥药理作用。L-PGDS在脂肪肝疾病中起着至关重要的作用,但其机制调控尚不清楚。因此,我们旨在利用棕榈酸盐诱导的细胞MASLD模型研究L-PGDS的调控机制。我们在棕榈酸盐处理的HepG2细胞中成功重现了MASLD表型。我们的研究结果显示了显著的脂质积累和脂质相关蛋白和基因表达的增加,以及棕榈酸盐浓度依赖性的L-PGDS下调。为了研究L-PGDS的下调,我们分别使用MG132、氯喹和环己亚胺来评估蛋白酶体降解、自噬和翻译活性。我们的基因和蛋白表达数据表明,L-PGDS下调的可能原因是抑制转录和随后的翻译。此外,我们的自噬结果也显示了LPGDS下调的作用。综上所述,可以得出结论,棕榈酸盐处理下调了L-PGDS,可能涉及转录-翻译和/或自噬途径。然而,需要进一步的研究来描述精确的分子机制,并将这些知识应用于MASLD的发病机制和治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
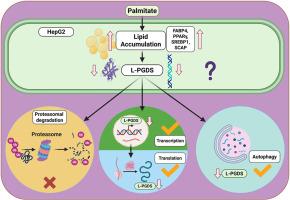
Palmitate-induced downregulation of lipocalin prostaglandin D2 synthase accompanies hepatic lipid accumulation in HepG2 cells
Metabolic dysfunction-associated steatotic liver disease (MASLD) is associated with multiple metabolic dysfunctions and poses a significant global health challenge. Our prior in vivo studies demonstrated that the absence of lipocalin prostaglandin D2 synthase (L-PGDS) leads to the development of fatty liver disease, and L-PGDS expression significantly decreased when C57BL/6 mice were kept on a high-fat diet. Briefly, L-PGDS belongs to the arachidonic acid pathway and enzymatically isomerizes prostaglandin H2 to prostaglandin D2, which imparts pharmacological effects via two receptors called DP1 and DP2. L-PGDS is an essential key player in fatty liver disease, but its mechanistic regulation still remains unknown. Therefore, we aimed to study the mechanistic regulation of L-PGDS using a palmitate-induced cellular MASLD model. We successfully recapitulated the MASLD phenotype in HepG2 cells with palmitate treatment. Our results showed significant lipid accumulation and increased lipidassociated protein and gene expression, along with palmitate concentration-dependent L-PGDS downregulation. To study the L-PGDS downregulation, we employed MG132, chloroquine, and cycloheximide to assess proteasomal degradation, autophagy, and translational activity, respectively. Our gene and protein expression data suggested the possible reason for L-PGDS downregulation via inhibiting transcription and subsequently translation. Additionally, our autophagy results also showed a role in LPGDS downregulation. In summary, it can be concluded that palmitate treatment downregulated L-PGDS, possibly involving transcription-translation and/or autophagy pathways. However, further studies are needed to delineate the precise molecular mechanism and apply this knowledge to MASLD pathogenesis and treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


