气液界面培养人支气管上皮细胞在transwell膜上的分化及组织学分析
IF 1.9
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
本方案描述了一个可重复和优化的工作流程,用于在Transwell膜上使用气液界面(ALI)培养培养的原代正常人支气管上皮细胞(NHBECs)的分化和组织学分析。该方法首先在浸没条件下扩展早期传代NHBECs,然后在胶原包被的Transwell插入物上播种。到达汇合后,使用PneumaCult™-ALI维持培养基将细胞转入ALI状态21-28天,促进粘膜纤毛分化。分化后,固定膜,在分级蔗糖溶液中冷冻保护,包埋在最佳切割温度(OCT)化合物中,然后冷冻切片。切片随后用苏木精和伊红(H&;E)染色以评估上皮形态,或周期性酸-希夫(PAS)染色以观察含有多糖的结构,如粘蛋白。该方案支持在Transwell模型上对NHBEC分化进行详细的结构和组织学评估,这对气道上皮生物学、粘膜纤毛功能和吸入毒理学的研究有价值。•胶原涂层Transwell插入物上NHBECs的ALI分化。•固定、蔗糖冷冻保护和OCT包埋用于冷冻切片。•H&;E和PAS染色用于可视化上皮细胞和粘蛋白。本文章由计算机程序翻译,如有差异,请以英文原文为准。
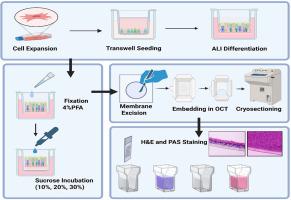
Differentiation and histological analysis of primary human bronchial epithelial cells cultured on transwell membranes using air-liquid interface culture
This protocol describes a reproducible and optimized workflow for the differentiation and histological analysis of primary normal human bronchial epithelial cells (NHBECs) cultured on Transwell membranes using air-liquid interface (ALI) culture. The method begins with expansion of early-passage NHBECs under submerged conditions, followed by seeding on collagen-coated Transwell inserts. Upon reaching confluence, cells are transitioned to ALI conditions using PneumaCult™-ALI maintenance medium for 21–28 days, promoting mucociliary differentiation. After differentiation, membranes are fixed, cryoprotected in graded sucrose solutions, embedded in Optimal Cutting Temperature (OCT) compound, and then cryosectioned. Sections are subsequently stained using hematoxylin and eosin (H&E) to assess epithelial morphology, or periodic acid–Schiff (PAS) staining to visualize polysaccharide-containing structures such as mucins. This protocol supports the detailed structural and histological evaluation of NHBEC differentiation on a Transwell model, which is valuable for studies of airway epithelial biology, mucociliary function, and inhalation toxicology.
- •ALI differentiation of NHBECs on collagen-coated Transwell inserts.
- •Fixation, sucrose cryoprotection, and OCT embedding for cryosectioning.
- •H&E and PAS staining for visualization of epithelial cells and mucin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


