吉妥珠单抗在一线治疗CBF-AML:来自回顾性多中心分析的见解
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
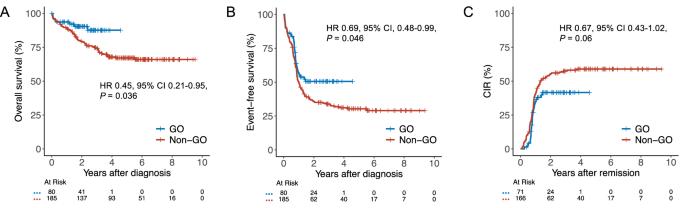
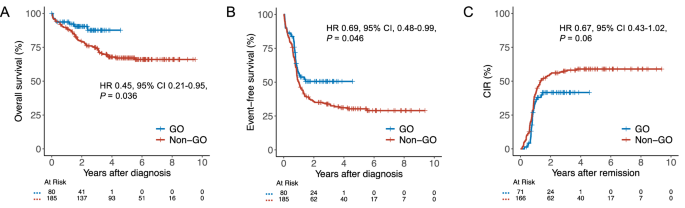
在强化化疗(IC)中加入吉妥珠单抗ozogamicin (GO)已成为治疗核心结合因子急性髓系白血病(CBF-AML)患者的主要方法。然而,氧化石墨烯在这一特定亚组中的有效性的证据主要基于十多年前进行的不同试验的荟萃分析数据。在这项基于注册表的研究中,我们评估了来自SAL、AMLCG和CELL合作研究组的265名CBF-AML患者将氧化石墨烯加入IC的影响。接受氧化石墨烯治疗的患者2年总生存率为90%,而未接受氧化石墨烯治疗的患者为80%(风险比[HR] 0.45, 95%可信区间[CI] 0.21-0.95, P = 0.036), 2年无事件生存率为51%,而未接受氧化石墨烯治疗的患者为36%(风险比[HR] 0.69, 95% CI 0.48-0.99, P = 0.046)。虽然氧化石墨烯与非氧化石墨烯患者的完全缓解率相当(89%对90%,P = 0.81),但更多的氧化石墨烯患者获得了可测量的残留疾病阴性缓解(77%对49%,P < 0.001),导致累积复发率降低(HR 0.67, 95% CI 0.43-1.02, P = 0.06)。尽管血小板恢复延迟,但氧化石墨烯治疗患者的高级别毒性并未增加。这些发现支持将氧化石墨烯纳入符合ic条件的CBF-AML患者的治疗方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gemtuzumab ozogamicin in first-line treatment of CBF-AML: insights from a retrospective multi-center analysis
The addition of gemtuzumab ozogamicin (GO) to intensive chemotherapy (IC) has become a mainstay in treating patients with core binding factor acute myeloid leukemia (CBF-AML). However, evidence for the efficacy of GO in this particular subgroup is primarily based on meta-analytic data from different trials conducted more than a decade ago. In this registry-based study, we evaluated the impact of adding GO to IC in 265 CBF-AML patients from the SAL, AMLCG, and CELL cooperative study groups. Patients receiving GO had a 2-year overall survival of 90% compared with 80% in those without GO (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.21–0.95, P = 0.036) and a 2-year event-free survival of 51% versus 36% (HR 0.69, 95% CI 0.48–0.99, P = 0.046). While complete remission rates in GO vs. non-GO patients were comparable (89% vs. 90%, P = 0.81), more GO patients achieved measurable residual disease-negative remission (77% vs. 49%, P < 0.001), resulting in numerically reduced cumulative incidence of relapse (HR 0.67, 95% CI 0.43–1.02, P = 0.06). Despite delayed platelet recovery, high-grade toxicities were not increased in GO-treated patients. These findings support the integration of GO into treatment protocols for IC-eligible patients with CBF-AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


