Rad23B通过异型缓冲延迟Ataxin-3液相到固相转变。
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Ataxin-3中聚谷氨酰胺(polyQ)长度的异常扩张与Machado-Joseph病有关,在神经元中形成核包涵体。最近,一种具有两个泛素相互作用基序(UIMs)的Ataxin-3的截断变体被证明可以进行液-液相分离(LLPS)。然而,Ataxin-3通过淀粉样蛋白形成和相分离聚集的分子机制尚不清楚。在这里,我们研究了在大脑中发现的最丰富的三种亚型Ataxin-3的LLPS特性。ataxin - 3q25形成相分离的液滴,经过老化,产生淀粉样纤维结构。PolyQ扩展的Q54形成短寿命的液滴,表现出快速成熟。Ataxin-3 c末端片段形成独特的直的,未分枝的淀粉样蛋白原纤维,显示可忽略不计的硫黄素-t结合。Rad23B是一种已知的Ataxin-3相互作用因子,是驱动蛋白酶体凝聚物形成的主要成分。我们发现与Rad23B的异型相互作用抑制Ataxin-3液滴成熟,但在稀释条件下不抑制淀粉样蛋白的形成,这表明Ataxin-3通过错误折叠途径聚集不同于冷凝途径。最后,我们发现Ataxin-3在亚砷酸盐胁迫下被整合到液体状的应力颗粒中,揭示了其在聚集动力学和胁迫响应中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
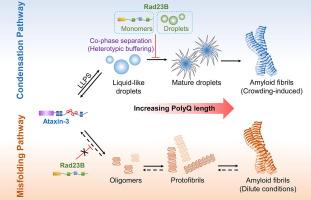
Rad23B Delays Ataxin-3 Liquid-to-solid Phase Transition Through Heterotypic Buffering
The abnormal expansion of polyglutamine (polyQ) length in Ataxin-3 is associated with Machado-Joseph disease, forming nuclear inclusions in neurons. A truncated variant of Ataxin-3 with two ubiquitin-interacting motifs (UIMs) has recently been shown to undergo liquid–liquid phase separation (LLPS). However, the molecular mechanisms underlying Ataxin-3 aggregation through amyloid formation and phase separation remain unclear. Here we investigated the LLPS properties of Ataxin-3 with three UIMs, the most abundant isoform found in the brain. Ataxin-3 Q25 forms phase-separated droplets which undergo aging, producing amyloid-like fibrillar structures. PolyQ expanded Q54 forms short-lived droplets that exhibit rapid maturation. Ataxin-3 C-terminal fragment forms unique straight, unbranched amyloid fibrils which display negligible Thioflavin-T binding. Rad23B, the main constituent driving the formation of proteasomal condensates, is a known Ataxin-3 interactor. We found that heterotypic interactions with Rad23B inhibit Ataxin-3 droplet maturation but do not inhibit amyloid formation under dilute conditions, suggesting that Ataxin-3 aggregation via misfolding pathway is distinct from condensation pathway. Finally, we show that Ataxin-3 is incorporated into liquid-like stress granules under arsenite stress, shedding light on its roles in aggregation dynamics and stress responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


