西布曲明类似物的安全性药理学评价与体外生物靶点筛选。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
许多作为食品和膳食补充剂销售的减肥产品都掺入了西布曲明的结构修改版本(类似物),西布曲明是一种减肥药,由于对心脏和神经系统有不利影响而退出市场。与西布曲明不同,其类似物缺乏体外和体内安全性数据。因此,为了确定西布曲明类似物的潜在健康影响,预测和测量了西布曲明类似物与45个与心脏、神经系统和其他器官相关的安全相关生物靶点(如受体、离子通道、转运体和酶)之间的结合。基于定量构效关系(QSAR)模型在计算机上预测靶结合浓度(Ki),并通过竞争配体结合实验在体外测量。血清素(SERT)、去甲肾上腺素(NET)和多巴胺(DAT)的转运体在体外和计算机上的Ki值非常接近,这些转运体与心脏和神经系统的不良健康影响有关。氯基、同属和去甲基西布曲明类似物表现出相似的结合谱,特别是与SERT、NET和DAT的有效结合(Ki, 9-403 nM)。然而,尽管化合物之间结构相似,但苯甲酰和甲酰类似物对几乎所有评估目标的结合都较弱(Ki, 0.447至bbb10 μM)。此外,对于选定的类似物,预测了代谢产物的靶结合;大多数代谢物(70%)与其各自的母体化学物质表现出相似的结合效力(Ki在10倍之内),这表明它们也可能对健康产生潜在影响。总的来说,生物靶点结合谱说明了西布曲明类似物之间重要的结构-活性关系,可以帮助识别潜在的不良健康影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
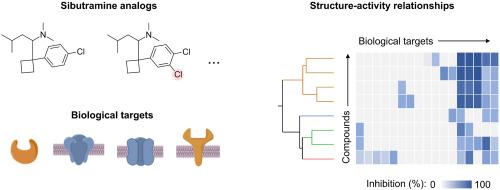
Safety pharmacology of sibutramine analogues evaluated by in silico and in vitro biological target screening
Many weight loss products marketed as foods and dietary supplements are adulterated with structurally modified versions (analogues) of sibutramine, a weight loss drug withdrawn from the market due to adverse effects in the heart and nervous system. Unlike sibutramine, its analogues lack in vitro and in vivo safety data. Therefore, to identify potential health effects of sibutramine analogues, binding was predicted and measured between sibutramine analogues and a panel of 45 safety-related biological targets (e.g., receptors, ion channels, transporters, and enzymes) related to the heart, nervous system, and other organs. Target binding concentrations (Ki) were predicted in silico based upon quantitative structure-activity relationship (QSAR) models and measured in vitro by competitive ligand binding assays. The in vitro and in silico Ki values closely agreed for transporters of serotonin (SERT), norepinephrine (NET), and dopamine (DAT), which are linked to adverse health effects in the heart and nervous system. The chloro, homo, and desmethylsibutramine analogues exhibited similar binding profiles and particularly potent binding to SERT, NET and DAT (Ki, 9–403 nM). However, despite structural similarity among the compounds, benzyl and formyl analogues exhibited weaker binding to nearly all targets evaluated (Ki, 0.447 to >10 μM). Additionally, for selected analogues, target binding was predicted for metabolites; a majority of metabolites (70 %) exhibited similar binding potency (Ki within 10-fold) to their respective parent chemicals, suggesting they may also contribute to potential health effects. Overall, biological target binding profiles illustrate important structure-activity relationships among sibutramine analogues that can help identify potential adverse health effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


