工程b7 - h3靶向VHH-Fc融合抗体在胰腺癌异种移植模型中显示了快速的肿瘤积累,用于红外成像
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
B7同源3蛋白(B7- h3)是肿瘤诊断和治疗的重要靶点。传统的b7 - h3靶向药物主要使用IgG抗体,由于其大分子大小(160-180 kDa,包括二聚体Fab-Fc结构),其在肿瘤中积累缓慢,导致外周副作用的风险增加。本研究开发了一种新型融合抗体A052,该抗体整合了纳米体重链(VHH)的可变结构域和IgG1的Fc结构域。这种设计保留了抗体的基本结构,同时将分子量降低到82 kDa。通过酶联免疫吸附法、流式细胞术和生物层干涉法的评估显示,与目前临床试验中的抗b7 - h3 IgG抗体hBRCA84D (KD = 8.13 nM)相比,A052的结合亲和力(KD = 77.2 pM)显著提高。为了比较A052和hBRCA84D在体内的肿瘤积累特性,我们用Cy5标记A052和hBRCA84D,并在PANC-1异种移植模型中使用红外成像进行评估。A052- cy5表现出更快的肿瘤积累,在注射后3小时达到可见肿瘤背景比(TBR),在24小时达到峰值1.97,并在3至144小时保持在1.50以上。相比之下,hBRCA84D-Cy5在48至110小时之间的TBR仅为1.5。这些结果表明,VHH-Fc融合抗体,如A052,为b7 - h3靶向治疗提供了一种新的方法,可能促进肿瘤快速分布,同时降低外周毒性风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
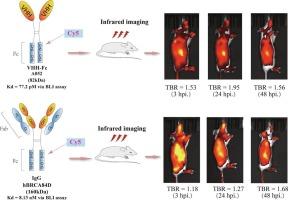
Engineered B7-H3-targeted VHH-Fc fusion antibody demonstrates rapid tumor accumulation for infrared imaging in pancreatic cancer xenograft model
B7 homolog 3 protein (B7-H3) represents a promising target for cancer diagnosis and therapy. Conventional B7-H3-targeting agents predominantly use IgG antibodies, which accumulate in tumors slowly due to their large molecular size (160–180 kDa, comprising a dimeric Fab-Fc structure), leading to an increased risk of peripheral side effects. In this study, a novel fusion antibody, A052, was developed, integrating the variable domain of a nanobody heavy chain (VHH) and the Fc domain of IgG1. This design preserves the fundamental structure of an antibody while reducing the molecular weight to 82 kDa. Evaluation through enzyme-linked immunosorbent assay, flow cytometry, and bio-layer interferometry revealed that A052 binds with a significantly higher affinity (KD = 77.2 pM) compared to the anti-B7-H3 IgG antibody, hBRCA84D, currently in clinical trials (KD = 8.13 nM). To compare the in vivo tumor accumulation properties, both A052 and hBRCA84D were labeled with Cy5 and assessed in a PANC-1 xenograft model using infrared imaging. A052-Cy5 showed faster tumor accumulation, reaching a visible tumor-background ratio (TBR) at 3 h post-injection, peaking at 1.97 at 24 h, and maintaining a TBR above 1.50 from 3 to 144 h. In contrast, hBRCA84D-Cy5 exhibited a TBR of 1.5 only between 48 and 110 h. These results suggest that VHH-Fc fusion antibodies, such as A052, offer a novel approach for B7-H3-targeted therapies, potentially facilitating rapid tumor distribution while minimizing peripheral toxicity risks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


