唑尼沙胺调节胆碱能标记物并缓解左旋多巴诱导的帕金森病大鼠运动障碍
IF 4.2
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
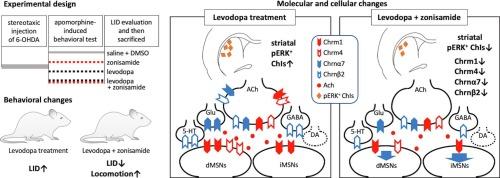
纹状体胆碱能中间神经元(ChIs)在基底神经节回路中起着关键的调节作用,并且越来越多地被认为是左旋多巴诱导的运动障碍(LID)在帕金森病(PD)中发展和表达的因素。我们的目的是研究唑尼沙胺(ZNS)是否显示出胆碱能系统对抗运动障碍作用的潜在贡献。用左旋多巴和/或ZNS治疗单侧PD模型大鼠。治疗2周后,评估LID严重程度,并采用实时RT-PCR分析纹状体毒碱M1 (Chrm1)和M4 (Chrm4)受体、烟碱α7 (Chrnα7)和β2 (Chrnβ2)亚基以及促啡肽(Pdyn)和促啡肽(Penk) mRNA表达水平。此外,采用免疫组织化学方法观察纹状体磷酸化细胞外信号调节激酶(pERK)阳性ChIs的比例。ZNS组(Z组)和生理盐水+ dmso组(N组)大鼠无LID,左旋多巴组(I组)大鼠有明显的LID。与ⅰ组相比,左旋多巴和ZNS联合使用(IZ组)大鼠的LID明显减弱,运动活性增加。N组和ⅰ组Chrm1、Chrm4、Chrnα7和Chrnβ2受体mRNA水平保持不变。相反,Z组Chrm1、Chrm4和Chrnβ2受体mRNA水平降低,而IZ组所有受体mRNA水平均下调。此外,纹状体perk阳性ChIs的比例在I组显著增加,而在IZ组则明显减少。这些发现表明,ZNS可能是一种双重治疗,可能通过抑制纹状体ChI过度活跃和下调乙酰胆碱受体表达来缓解LID,同时维持运动功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zonisamide modulates cholinergic markers and alleviates levodopa-induced dyskinesia in a rat model of Parkinson's disease
Striatal cholinergic interneurons (ChIs) play a key modulatory role in basal ganglia circuits and is increasingly recognized as contributors to levodopa-induced dyskinesia (LID) development and expression in Parkinson's disease (PD). We aimed to investigate whether zonisamide (ZNS) exhibits the potential contribution of the cholinergic system to the antidyskinetic effects.
Unilateral PD model rats were treated with levodopa and/or ZNS. Two weeks post-treatment, LID severity was assessed, and striatal mRNA expression levels for muscarinic M1 (Chrm1) and M4 (Chrm4) receptors, and nicotinic α7 (Chrnα7) and β2 (Chrnβ2) subunits, as well as prodynorphin (Pdyn) and proenkephalin (Penk), were analyzed using real-time RT-PCR. Additionally, the proportion of striatal phosphorylated extracellular signal-regulated kinase (pERK)-positive ChIs was observed using immunohistochemistry.
LID was absent in ZNS (Group Z) or saline + DMSO-treated (Group N) rats but pronounced in levodopa-treated rats (Group I). Rats receiving both levodopa and ZNS (Group IZ) showed less-pronounced LID and increased locomotive activity compared with Group I. Chrm1, Chrm4, Chrnα7, and Chrnβ2 receptor mRNA levels remained unchanged in Groups N and I. Conversely, Chrm1, Chrm4, and Chrnβ2 receptor mRNA levels were reduced in Group Z, whereas all receptor mRNAs were downregulated in Group IZ. Additionally, the proportion of striatal pERK-positive ChIs significantly increased in Group I, whereas its reduction was observed in Group IZ.
These findings suggest that ZNS may serve as a dual-purpose therapy by potentially alleviating LID while maintaining locomotor function, possibly through the suppression of striatal ChI overactivity and downregulation of acetylcholine receptor expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


