一系列N2O2配位旋光性氨基醇受体中性铜(II)配合物的合成、结构和光谱研究
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
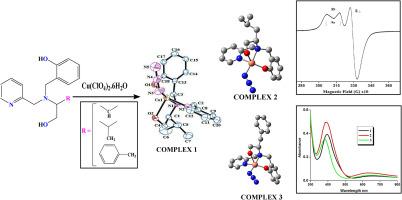
本研究的重点是利用实验和理论相结合的方法合成和表征一系列三种旋光性氨基醇还原希夫碱铜(II)配合物。通过元素分析、1H NMR、IR、ESI-MS和UV-vis光谱等多种光谱技术对合成的配体和配合物进行了表征。此外,还报道了一个配合物的x射线结构。优化结果表明,所研究的配合物中的Cu(II)中心具有畸变的方锥体几何形状(τ值约为0.3)。此外,利用随时间密度泛函理论计算了Cu(II)配合物在溶液中的电子激发能。采用TDDFT方法分析了在配合物中观察到的高强度和高分辨波段的性质。通过循环伏安法研究了配合物的电化学行为,发现所有配合物的电化学行为相似。它们在−0.5至+1.5 V的电压范围内表现出一对电流峰值。顺磁配合物在77 K的men中进行了EPR谱分析,结果表明,五配位Cu(II)配合物的EPR谱显示出围绕金属中心的2B1g方锥体配位的轴对称体系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, structure and spectroscopic studies of a series of neutral cu(II) complexes of N2O2 coordinating optically active amino alcohol receptor
This work focuses on the synthesis and characterization of a series of three optically active amino alcohol reduced Schiff base Cu(II) complexes using a combined experimental and theoretical approach. The synthesized ligands and complexes were characterized through various spectroscopic techniques, including elemental analysis, 1H NMR, IR, ESI-MS, and UV–vis spectroscopy. Additionally, the X-ray structure of one complex has been reported. The optimization results indicate that the Cu(II) center in the studied complexes has a distorted square pyramidal geometry (with a τ value of approximately 0.3). Furthermore, the electronic excitation energies of the Cu(II) complexes were calculated using time-dependent density functional theory in the solution phase. The TDDFT method was employed to analyze the nature of the highly intense and well-resolved bands observed in the complexes. The electrochemical behaviors of the complexes were investigated through cyclic voltammetry, revealing similar behavior among all the complexes. They exhibited a pair of current peaks in the voltage range of −0.5 to +1.5 V. The paramagnetic complexes were subjected to EPR spectroscopy at 77 K in MeCN, which showed an axial symmetric system (2B1g) of square pyramidal coordination around the metal center in the EPR spectra of the pentacoordinated Cu(II) complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


