DDB1通过增强pak1介导的有氧糖酵解促进垂体腺瘤进展。
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
DNA损伤结合蛋白1 (DNA damage binding protein 1, DDB1)是CUL4-DDB1 E3连接酶的底物受体,在多种癌症中过表达,是肿瘤促进因子。然而,DDB1在垂体腺瘤(PA)代谢中的作用尚不清楚。本研究发现,DDB1在PA组织中表达上调,沉默DDB1可显著抑制PA细胞的增殖、细胞周期、泌乳素(PRL)分泌和有氧糖酵解,而过表达DDB1则具有相反的作用。我们还通过Co-IP和双免疫荧光实验证实了DDB1和PAK1的共定位和相互作用。进一步的细胞功能分析表明,PAK1敲低或2DG处理逆转了DDB1过表达对PA细胞系增殖、细胞周期、PRL分泌、有氧糖酵解和凋亡的影响。重要的是,我们观察到体内DDB1的过表达促进了异种移植小鼠的肿瘤生长,这可以通过敲除PAK1来逆转。简而言之,DDB1通过增强pak1介导的有氧糖酵解促进PA肿瘤生长和PRL分泌。我们的研究结果表明,靶向DDB1/PAK1轴可能为PA提供一种潜在的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
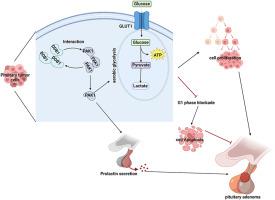
DDB1 promotes pituitary adenoma progression by enhancing PAK1-mediated aerobic glycolysis
DNA damage binding protein 1 (DDB1), a substrate receptor of CUL4-DDB1 E3 ligase, is overexpressed in various cancers and acts as a tumor promoting factor. However, the role of DDB1 in the metabolism of pituitary adenoma (PA) remains unclear. Here, we found that DDB1 was upregulated in PA tissues, and silencing DDB1 significantly inhibited the proliferation, cell cycle, prolactin (PRL) secretion and aerobic glycolysis in PA cells, whereas DDB1 overexpression had the opposite effect. We also confirmed that the co-localization and interaction of DDB1 and PAK1 using Co-IP and dual immunofluorescence experiments. Further analysis of cell functions showed that PAK1 knockdown or 2DG treatment reversed the effect of DDB1 overexpression on proliferation, cell cycle, PRL secretion, aerobic glycolysis, and apoptosis in PA cell lines. Importantly, we observed that overexpression of DDB1 in vivo promoted tumor growth in xenograft mice, which could be reversed by knockout of PAK1. In short, DDB1 promotes PA tumor growth and PRL secretion by enhancing PAK1-mediated aerobic glycolysis. Our results reveal that targeting the DDB1/PAK1 axis may provide a potential therapeutic strategy for PA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


