以患者为中心的医疗工具在心力衰竭患者心脏康复中的持续激励:一项多中心随机对照试验方案(EXERCISE-HF试验)
IF 1.4
Q4 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
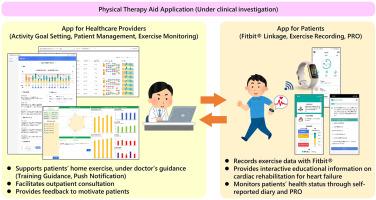
心力衰竭严重影响患者的生活质量,并造成显著的社会和经济负担。尽管心脏康复具有有益的效果,但全球的心脏康复参与率仍然很低。可穿戴生物识别技术的最新进展可以通过实时监测和个性化反馈来提高患者的依从性。本研究旨在开发和评估一项运动支持计划,该计划整合了可穿戴设备,以提高心力衰竭患者的康复效果。方法根据患者和医生的反馈,结合可穿戴设备开发一种创新实用的心脏康复方案。一项多中心随机对照临床试验旨在评估2022年10月至2025年1月在日本心力衰竭患者中应用的安全性和有效性。与传统的运动监测应用程序相比,开发的应用程序提供了一系列旨在促进患者参与和促进长期坚持的功能。这些功能包括:(1)个性化目标设定,(2)与医疗保健提供者直接沟通,(3)通过教学视频进行心力衰竭教育,(4)自动动机反馈,以及(5)心脏康复和自我保健研究总结的策划图书馆。符合纳入标准的患者(包括临床诊断为心力衰竭的年龄≥18岁的患者)将被随机分配到以下两组中的一组:综合运动支持应用程序组或标准护理组,其中仅实施可穿戴设备。根据基线和分配因素调整后,12周时峰值VO2的变化将作为主要终点进行分析。次要结局包括生活质量测量和再住院率。数据分析将遵循意向治疗原则,结果报告为双尾95%置信区间和相应的p值。本研究已获得相关机构伦理委员会批准(批准号:DB23-001;iRCT: 2032230388)。在参与研究之前,将获得所有参与者的知情同意。这项研究的结果将通过同行评议的出版物传播,并在有关的科学会议上提出。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Patient-centered medical tools for sustained motivation in cardiac rehabilitation of patients with heart failure: protocol of a multicenter randomized controlled trial (EXERCISE-HF trial)
Introduction
Heart failure substantially affects the quality of life of patients and imposes notable social and economic burdens. Despite the beneficial effects of cardiac rehabilitation, global participation rates remain low. Recent advances in wearable biometric technologies may improve patient adherence through real-time monitoring and personalized feedback. This study aimed to develop and evaluate an exercise-support program that integrates wearable devices to enhance rehabilitation outcomes in patients with heart failure.
Methods
An innovative and practical cardiac rehabilitation program combined with a wearable device was developed based on patients and physicians’ feedback. A multicenter randomized controlled clinical trial was designed to evaluate the safety and effectiveness of the application in patients with heart failure in Japan from October 2022 to January 2025. Compared with traditional exercise-monitoring applications, the developed application offers an array of features that are designed to foster patient engagement and promote long-term adherence. These features include (1) individualized goal setting, (2) direct communication with healthcare providers, (3) education on heart failure through instructional videos, (4) automated motivational feedback, and (5) a curated library of research summaries on cardiac rehabilitation and self-care. Patients who meet the inclusion criteria (including those aged ≥18 years with a clinical diagnosis of heart failure) will be randomly assigned to one of two groups as follows: the integrated exercise-support app group or the standard care group, in which only a wearable device was implemented. The change in peak VO2 at 12 weeks, adjusted for baseline and allocation factors, will be analyzed as the primary endpoint. The secondary outcomes include quality-of-life measures and re-hospitalization rates. Data analysis will follow the intention-to-treat principle, with results reported as two-tailed 95 % confidence intervals and corresponding p-values.
This study was approved by the relevant institutional ethics committee (approval number: DB23-001; iRCT: 2032230388). Informed consent will be obtained from all participants before study participation. The results of the study will be disseminated through peer-reviewed publications and presented at relevant scientific meetings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Contemporary Clinical Trials Communications
Pharmacology, Toxicology and Pharmaceutics-Pharmacology
CiteScore
2.70
自引率
6.70%
发文量
146
审稿时长
20 weeks
期刊介绍:
Contemporary Clinical Trials Communications is an international peer reviewed open access journal that publishes articles pertaining to all aspects of clinical trials, including, but not limited to, design, conduct, analysis, regulation and ethics. Manuscripts submitted should appeal to a readership drawn from a wide range of disciplines including medicine, life science, pharmaceutical science, biostatistics, epidemiology, computer science, management science, behavioral science, and bioethics. Contemporary Clinical Trials Communications is unique in that it is outside the confines of disease specifications, and it strives to increase the transparency of medical research and reduce publication bias by publishing scientifically valid original research findings irrespective of their perceived importance, significance or impact. Both randomized and non-randomized trials are within the scope of the Journal. Some common topics include trial design rationale and methods, operational methodologies and challenges, and positive and negative trial results. In addition to original research, the Journal also welcomes other types of communications including, but are not limited to, methodology reviews, perspectives and discussions. Through timely dissemination of advances in clinical trials, the goal of Contemporary Clinical Trials Communications is to serve as a platform to enhance the communication and collaboration within the global clinical trials community that ultimately advances this field of research for the benefit of patients.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


