发现基于1,8-萘酰亚胺的新型硫脲苯磺酰胺衍生物作为碳酸酐酶IX抑制剂,诱导铁下垂并抑制三阴性乳腺癌转移
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
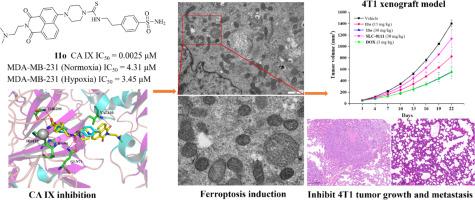
碳酸酐酶IX (CAIX)是许多缺氧肿瘤治疗干预的一个有吸引力的靶点。本文中,我们描述了基于1,8-萘酰亚胺的新型硫脲苯磺酰胺衍生物作为碳酸酐酶IX抑制剂的发现,这些抑制剂可诱导铁下垂并抑制三阴性乳腺癌转移。其中具有代表性的化合物110有效地抑制了CA IX酶活性,对CA IX具有较高的选择性。通过分子对接研究和分子动力学模拟,深入了解110在CAIX结合口袋中的结合相互作用和配合物的稳定性。令人满意的是,该化合物在缺氧条件下对MDA-MB-231细胞的抗肿瘤活性优于常氧条件下的抗肿瘤活性,并优于参比化合物SLC-0111。机制研究表明,110能有效抑制MDA-MB-231细胞拓扑异构酶I活性,诱导细胞凋亡和铁下垂,抑制细胞迁移。值得注意的是,体内实验结果表明,110在高转移性小鼠乳腺癌4 T1细胞的异种移植模型中具有有效的抗肿瘤活性和显著的抗转移效力。这些发现表明110可能是对抗三阴性乳腺癌转移的潜在候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of novel thiourea benzenesulfonamides based 1,8-naphthalimide derivatives as carbonic anhydrase IX inhibitors that induce ferroptosis and inhibit triple-negative breast cancer metastasis
Carbonic anhydrase IX (CAIX) is an attractive target for therapeutic intervention in many hypoxic tumors. Herein, we described the discovery of novel thiourea benzenesulfonamides based 1,8-naphthalimide derivatives as carbonic anhydrase IX inhibitors that induced ferroptosis and inhibited triple-negative breast cancer metastasis. One of the representative compounds, 11o, effectively inhibited CA IX enzymatic activity and displayed high selective for CA IX over CA II. Molecular docking study and molecular dynamics simulations were also performed to gain insights into the binding interactions of 11o in the binding pocket of CAIX and complex stability. Satisfyingly, this compound exhibited superior antitumor activities against MDA-MB-231 cells under hypoxia than normoxic conditions and surpassed reference compound SLC-0111. Mechanism studies revealed that 11o effectively inhibited topoisomerase I activity, induced cell apoptosis and ferroptosis and suppressed cell migration in MDA-MB-231 cells. Notably, in vivo assays results demonstrated that 11o exerted efficient antitumor activity and significant anti-metastasis potency in a xenograft model of highly metastatic murine breast cancer 4 T1 cells. These findings suggest that 11o may serve as a potential candidate for combating triple-negative breast cancer metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


