5-氮胞苷和地西他滨在小鼠和人细胞中均可诱导c>g转化
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
5-氮杂胞苷(5AZA)是一种DNA甲基转移酶抑制剂(DNMTi),临床上用于治疗骨髓增生异常肿瘤(MDS),并在适应症外用于包括急性髓系白血病在内的许多恶性肿瘤。这种胞苷类似物消耗细胞内DNMT1,并且已经假设DNMT1的消耗导致甲基化肿瘤抑制基因的低甲基化和去抑制。我们使用MDS临床前模型来研究5-氮杂胞苷的疗效。出乎意料的是,我们发现5AZA治疗小鼠急性淋巴性白血病(ALL)的发生率增加。全外显子组测序(WES)显示,在5AZA处理的小鼠中有大量的C >; G转录,包括已知对ALL很重要的基因,如Chd4、Ikzf1和Trp53。单碱基置换(SBS)分析显示,ALL细胞中C >; G突变增加,突变特征与先前描述的SBS39特征相似。在体外克隆扩增后鉴定的基因毒性突变特征GEMINI (genetoxic Mutational Signature Identified After Clonal Expansion in vitro)试验中发现,C >; G突变在小鼠和人类细胞系中都有所增加。此外,类似的GEMINI实验显示,地西他滨可诱导细胞中C >; G突变。综上所述,这些发现表明,氮杂核苷在体外和体内诱导C >; G突变,并与小鼠细胞的白血病转化有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
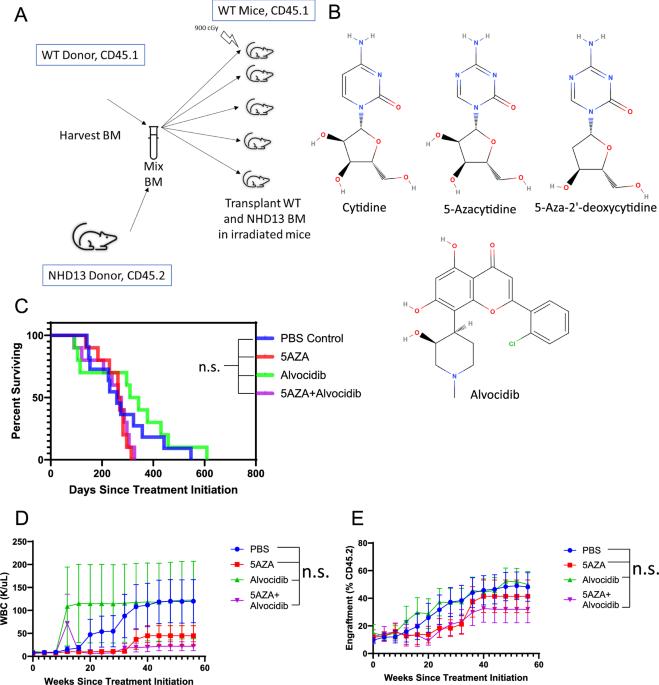
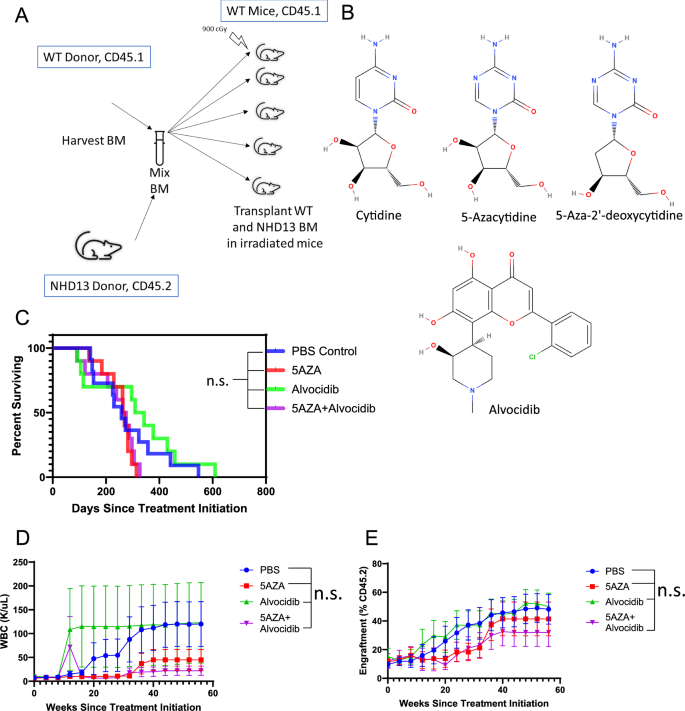
5-Azacytidine and decitabine induce C > G transversions in both murine and human cells
5-Azacytidine (5AZA) is a DNA methyltransferase inhibitor (DNMTi) used clinically to treat myelodysplastic neoplasm (MDS), and is used off-label for a number of malignancies including acute myeloid leukemia. This cytidine analog depletes intracellular DNMT1, and it has been hypothesized that DNMT1 depletion leads to hypomethylation and de-repression of methylated tumor suppressor genes. We used a pre-clinical model of MDS to investigate the efficacy of 5-azacytidine. Unexpectedly, we found an increased frequency of acute lymphoid leukemia (ALL) in 5AZA treated mice. Whole exome sequencing (WES) revealed a large number of C > G transversions in 5AZA treated mice, including genes known to be important for ALL such as Chd4, Ikzf1, and Trp53. Single base substitution (SBS) profiling revealed increased C > G mutations in the ALL cells, with a mutation signature similar to the previously described SBS39 signature. An in vitro GEMINI (Genotoxic Mutational Signature Identified After Clonal Expansion In vitro) assay recapitulated the finding of increased C > G mutations in both murine and human cell lines. Furthermore, similar GEMINI assays revealed induction of C > G mutations in cells treated with decitabine. Taken together, these findings demonstrate that azanucleosides induce C > G mutations both in vitro and in vivo, and are linked to leukemic transformation in murine cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


