类固醇受体共激活因子-1通过共激活NF-κB促进mettl3介导的m6A修饰,促进胶质母细胞瘤的恶性进展。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
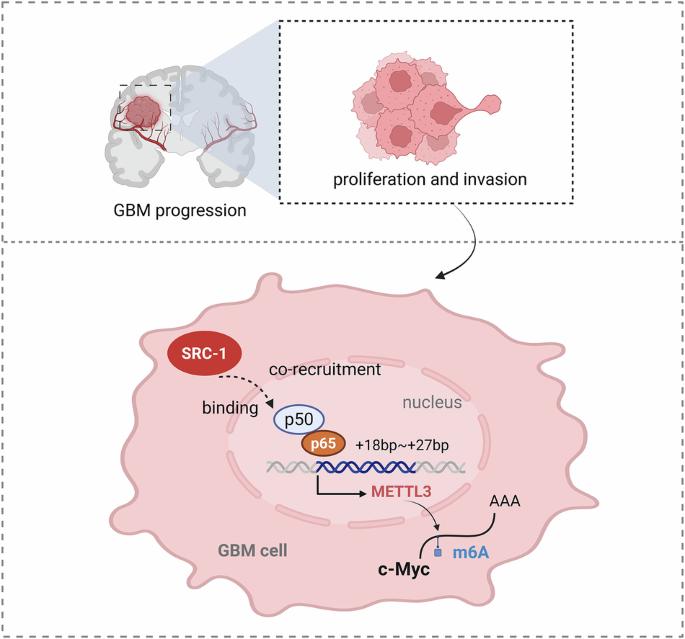
胶质母细胞瘤(GBM)是一种预后不良的不治之症。然而,类固醇受体共激活因子-1 (SRC-1)对n6 -甲基腺苷(m6A) RNA修饰的潜在影响及其在促进GBM恶性进展中的作用尚不清楚。利用CGGA数据库的数据分析SRC-1与m6A“writer”蛋白甲基转移酶3 (methyltransferase 3, METTL3)之间的关系。Dot blot和MeRIP-qPCR检测SRC-1敲低或过表达对GBM中m6A修饰水平的影响。通过体外研究SRC-1对GBM细胞增殖、迁移、细胞周期、集落形成和凋亡的影响以及体内移植裸鼠肿瘤体积/重量的影响,评估SRC-1在GBM中调控METTL3的生物学功能。随后进行Co-IP、免疫荧光、双荧光素酶和ChIP-qPCR检测。通过分析CGGA数据库,我们确定SRC-1与METTL3在GBM中有密切的正相关。SRC-1在GBM中显著增加m6A RNA修饰水平,SRC-1敲低通过抑制METTL3显著抑制c-Myc m6A甲基化和mRNA稳定性,SRC-1过表达通过增加METTL3导致高甲基化。SRC-1敲低可抑制体外GBM细胞的增殖、迁移、抗凋亡及S期和G2/M期。机械上,SRC-1与NF-κB p50/p65异源二聚体相互作用,其中p65通过直接结合其启动子的特定区域(+18至+27 bp)激活METTL3,从而增加c-Myc的m6A修饰,最终促进GBM进展。重要的是,SRC-1敲除和使用蟾毒灵(一种SRC抑制剂)治疗均可减少GBM的进展。总之,本研究首次提供了SRC-1通过结合NF-κB和调节mettl3介导的c-Myc的m6A修饰促进GBM进展的全面证据,为GBM的潜在治疗策略提供了新的见解。本研究揭示的机理示意图。SRC-1通过与NF-κB转录因子结合,调控mettl3介导的c-Myc的m6A RNA修饰,促进GBM的进展。在BioRender中创建。https://BioRender.com。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Steroid receptor coactivator-1 facilitates METTL3-mediated m6A modification by coactivating NF-κB and promotes the malignant progression of glioblastoma
Glioblastoma (GBM) is an incurable disease with a poor prognosis. However, the potential impact of steroid receptor coactivator-1 (SRC-1) on N6-methyladenosine (m6A) RNA modification and its role in promoting malignant progression in GBM remain unclear. The relationship between SRC-1 and the m6A “writer” protein, methyltransferase 3 (METTL3), was analyzed using data from the CGGA database. Dot blot and MeRIP‒qPCR were performed to evaluate the effects of SRC-1 knockdown or overexpression on the level of m6A modification in GBM. The biological functions of SRC-1 in regulating METTL3 in GBM were evaluated by assessing its effects on proliferation, migration, cell cycle, colony formation, and apoptosis in vitro and the tumor volume/weight of nude mice xenografted with GBM cells in vivo. Co-IP, immunofluorescence, dual-luciferase, and ChIP‒qPCR assays were subsequently conducted. By analyzing the CGGA database, we determined that SRC-1 has a close positive relationship with METTL3 in GBM. SRC-1 significantly increased the m6A RNA modification level in GBM, SRC-1 knockdown markedly inhibited c-Myc m6A methylation and mRNA stability by suppressing METTL3, and SRC-1 overexpression led to hypermethylation by increasing METTL3. SRC-1 knockdown inhibited the proliferation, migration, apoptosis resistance, and S and G2/M phases of GBM cells in vitro. Mechanically, SRC-1 interacted with the heterodimer of NF-κB p50/p65, whereby p65 activated METTL3 by directly binding to a specific region of its promoter (+18 to +27 bp), thereby increasing the m6A modification of c-Myc and ultimately promoting GBM progression. Importantly, both SRC-1 knockdown and treatment with bufalin, an SRC inhibitor, reduced GBM progression. In conclusion, this study provides the first comprehensive evidence that SRC-1 facilitates GBM progression by binding to NF-κB and regulating METTL3-mediated m6A modification of c-Myc, offering new insights into potential therapeutic strategies for GBM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


