CsAg4Br3-xI2+x超离子导体的晶体结构、稳定性和存在区域
IF 9.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
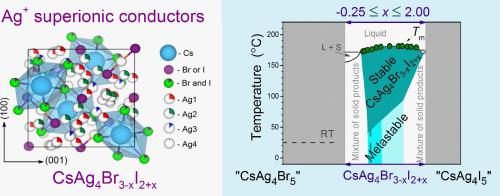
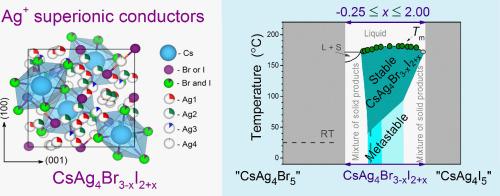
CsAg4Br3-xI2+x固溶体与众所周知的α-RbAg4I5超离子导体同构,具有非常相似的Ag离子导电性。然而,CsAg4Br3-xI2+x电解质作为一种很有前景的低温超离子导体,却很少得到研究。因此,本工作着手澄清它们的热力学稳定性,定义一个存在区域,并将晶体结构参数表征为x的函数。CsAg4Br3-xI2+x样品在x = -0.25到x = 2.00的广泛范围内通过固相合成制备。通过x射线衍射和热分析确定了样品的相组成。新合成的样品在0.25 <的组成范围内观察到单相的CsAg4Br3-xI2+x;X≤1.35;而所有固溶体CsAg4Br3-xI2+x(除x = 0.38外)在室温下均为亚稳态。虽然研究样品可以很容易地制备,但在25°C的条件下储存数月后,它们会逐渐分解。以碘为主的固溶体分解得最快,而以溴为主的固溶体分解得慢得多。只有一个研究样本(x = 0.38)没有发生变化;该样品在长达3年多的整个贮存期内保持单相。建立了银在新制备样品中不同晶体位置的占有与x的函数关系。四组分体系Cs - Ag - Br - I沿两个不存在的化合物“CsAg4Br5”-“CsAg4I5”之间的多热截面表示CsAg4Br3-xI2+x固溶体场。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Crystal structure, stability and existence region of CsAg4Br3-xI2+x superionic conductors
CsAg4Br3-xI2+x solid solutions, which are isomorphic to the well-known α-RbAg4I5 superionic conductor, exhibit very similar conductivity by Ag+ ions. However, the CsAg4Br3-xI2+x electrolytes, promising low-temperature superionic conductors, have been little studied. Therefore, the present work set out to clarify their thermodynamic stability, define an existence region, and characterise the crystal structure parameters as a function of x. CsAg4Br3-xI2+x samples were prepared by solid-phase synthesis over a wide range from x = –0.25 to x = 2.00. The phase composition of the samples was specified by X-ray diffraction and thermal analysis. Single-phase CsAg4Br3-xI2+x was observed for freshly synthesised samples within the composition range of 0.25 < x ≤ 1.35; however, all solid solutions CsAg4Br3-xI2+x (with the exception of x = 0.38) were found to be metastable at room temperature. Although the research samples can be easily prepared, they gradually decomposed when stored at 25 °C over a period of months. Solid solutions having a predominance of iodine decomposed most rapidly, while samples with a predominance of bromine decomposed much more slowly. Only one of the studied samples, having x = 0.38, underwent no changes; this sample remained single-phase throughout the entire storage period of up to >3 years. The occupancy of different crystallographic positions of Ag in freshly prepared samples as a function of x was established. The polythermal cross-section of the four-component system Cs – Ag – Br – I along the line between two non-existent compounds “CsAg4Br5” – “CsAg4I5” was used to represent the CsAg4Br3-xI2+x solid solution field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Materialia
工程技术-材料科学:综合
CiteScore
16.10
自引率
8.50%
发文量
801
审稿时长
53 days
期刊介绍:
Acta Materialia serves as a platform for publishing full-length, original papers and commissioned overviews that contribute to a profound understanding of the correlation between the processing, structure, and properties of inorganic materials. The journal seeks papers with high impact potential or those that significantly propel the field forward. The scope includes the atomic and molecular arrangements, chemical and electronic structures, and microstructure of materials, focusing on their mechanical or functional behavior across all length scales, including nanostructures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


