APOE缺乏触发肝癌巨噬细胞抗肿瘤活性。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
巨噬细胞浸润与肝癌患者预后不良及免疫治疗耐药相关。然而,由于肿瘤相关巨噬细胞(tam)固有的异质性,很难靶向它们。特定的TAM亚群可能在肿瘤发生中表现出不同的功能。在此,我们确定了以APOE表达升高为特征的TAM亚群,这与HCC患者的总生存率较低相关。APOE+ TAM强度在ICB无应答肿瘤中高度升高,且与CD8+ T细胞浸润呈负相关。途径分析和细胞相互作用揭示APOE+ tam通过信号整合和胆固醇外排抑制CD8+ T细胞。此外,巨噬细胞缺乏APOE可延缓肿瘤生长并促进CD8+ T细胞的浸润。通过免疫治疗抵抗小鼠模型,我们发现APOE阻断与抗pd -1治疗协同作用并抑制肿瘤生长。我们的研究结果阐明了APOE+ tam在免疫抑制微环境形成中的关键作用,并为ICB联合治疗提供了潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
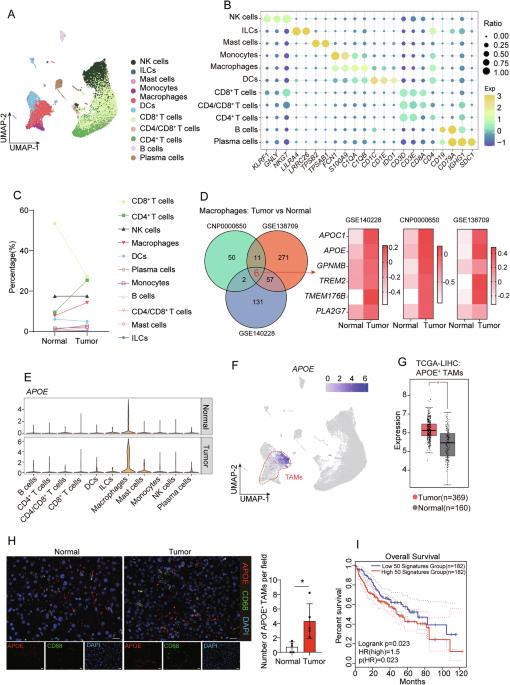
APOE deficiency triggers anti-tumour activity of macrophages in liver cancer
Macrophage infiltration correlates with poor prognosis in patients with liver cancer and resistance to immunotherapy. However, it is difficult to target tumour-associated macrophages (TAMs) because of their inherent heterogeneity. Specific TAM subsets may exhibit distinct functions in tumorigenesis. Herein, we identify a TAM subset characterised by elevated APOE expression, which is correlated with poor overall survival of patients with HCC. The APOE+ TAM intensity is highly elevated in ICB non-responder tumours and negatively correlated with CD8+ T cell infiltration. Pathway analysis and cell interaction reveal that APOE+ TAMs suppress CD8+ T cells through signal integration and cholesterol efflux. Furthermore, APOE deficiency in macrophages delays tumour growth and promotes the infiltration of CD8+ T cells. Using an immunotherapy-resistant mouse model, we showed that APOE blockade synergises with anti-PD-1 therapy and inhibits tumour growth. Our results elucidate the crucial role of APOE+ TAMs in the formation of immunosuppressive microenvironments and offer a potential therapeutic target for ICB combined therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


