瑞士儿童药物遗传学建议用药情况。
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
药物遗传学(PGx)越来越多地在成人人群中实施,但其在儿童中的潜力仍不确定。本研究的目的是利用2017年至2021年的Helsana索赔数据,调查瑞士儿童的PGx药物使用情况。我们从药物基因组学知识库中确定了82种具有与24个基因变异相关的儿科指南注释的药物。在159172名持续投保的儿童中,66.1%的儿童在研究期间至少服用了一种PGx药物。使用人数最多的三种PGx药物分别是全身布洛芬(59.1%)、昂丹司琼(8.3%)和局部氟尿嘧啶(7.5%)。超过96%的潜在药物-基因相互作用是由7个基因(CYP2C9、CYP2D6、DPYD、CYP2C19、MT-RNR1、CACNA1S和RYR1)引起的。在瑞士,有大量儿童声称服用了PGx药物,这意味着大量儿童可以从PGx检测中受益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
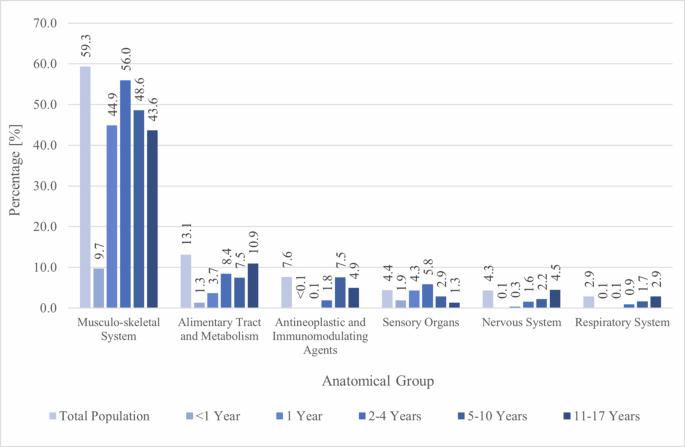
Utilisation of drugs with pharmacogenetic recommendations in children in Switzerland
Pharmacogenetics (PGx) is increasingly implemented in the adult population, but its potential in children remains uncertain. The aim of this study was to investigate PGx drug utilization in children in Switzerland, using Helsana claims data between 2017 and 2021. We identified 82 drugs with paediatric guideline annotations associated with variants in 24 genes from the Pharmacogenomics Knowledgebase. Of 159 172 children continuously insured, 66.1% claimed at least one PGx drug during the study period. The three PGx drugs with the highest user numbers were systemically administered ibuprofen (59.1%), ondansetron (8.3%), and locally administered fluorouracil (7.5%). Over 96% of all potential drug-gene interactions were caused by seven genes (CYP2C9, CYP2D6, DPYD, CYP2C19, MT-RNR1, CACNA1S, and RYR1). The high number of children claiming PGx drugs in Switzerland implies that a significant number of children could benefit from PGx testing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


