在肿瘤细胞和巨噬细胞之间的TMEM门道靶向CSF-1信号可抑制乳腺癌的传播。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肿瘤细胞内渗是转移性传播的必要条件,但其确切机制尚不完全清楚。我们之前的研究表明,在乳腺癌中,表达Mena的肿瘤细胞、Tie2hi/VEGFhi巨噬细胞和血管内皮细胞之间的直接和稳定的关联,为肿瘤细胞的内渗创造了一个门静脉,称为“肿瘤转移微环境”(TMEM)门静脉,导致肿瘤细胞向远处扩散。TMEM门道密度,又称TMEM门道评分,是临床验证的乳腺癌远处转移预后指标。尽管我们知道肿瘤细胞利用与TMEM门道相关的瞬时血管开口进行内渗,但涉及TMEM门道功能的确切信号机制仅部分被了解。通过两种乳腺癌小鼠模型和体外内渗实验,我们报道了TMEM门洞肿瘤细胞分泌的CSF-1刺激Tie2hi TMEM门洞巨噬细胞局部分泌VEGF-A,导致TMEM门洞相关内皮细胞之间的内皮连接解离,支持肿瘤细胞内渗。急性阻断CSF-1/CSF-1R信号可降低巨噬细胞VEGF-A的分泌、TMEM门道相关的血管开放、肿瘤细胞跨内皮迁移和播散。这些关于调节TMEM门道功能的信号事件的新见解应该作为转移性疾病的治疗策略进一步探索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
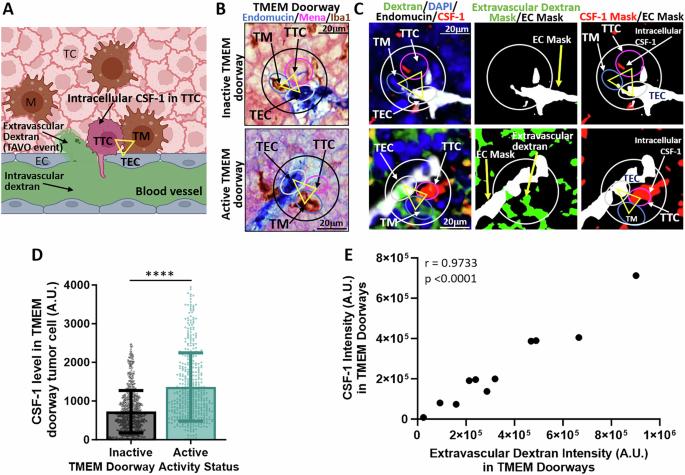
Targeting CSF-1 signaling between tumor cells and macrophages at TMEM doorways inhibits breast cancer dissemination
Tumor cell intravasation is essential for metastatic dissemination, but its exact mechanism is incompletely understood. We have previously shown that in breast cancer, the direct and stable association of a tumor cell expressing Mena, a Tie2hi/VEGFhi macrophage, and a vascular endothelial cell, creates an intravasation portal, called a “tumor microenvironment of metastasis” (TMEM) doorway, for tumor cell intravasation, leading to dissemination to distant sites. The density of TMEM doorways, also called TMEM doorway score, is a clinically validated prognostic marker of distant metastasis in breast cancer patients. Although we know that tumor cells utilize TMEM doorway-associated transient vascular openings to intravasate, the precise signaling mechanisms involved in TMEM doorway function are only partially understood. Using two mouse models of breast cancer and an in vitro assay of intravasation, we report that CSF-1 secreted by the TMEM doorway tumor cell stimulates local secretion of VEGF-A from the Tie2hi TMEM doorway macrophage, leading to the dissociation of endothelial junctions between TMEM doorway-associated endothelial cells, supporting tumor cell intravasation. Acute blockade of CSF-1/CSF-1R signaling decreases macrophage VEGF-A secretion as well as TMEM doorway-associated vascular opening, tumor cell trans-endothelial migration, and dissemination. These new insights into signaling events regulating TMEM doorway function should be explored further as treatment strategies for metastatic disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


