大鼠主动脉内皮糖萼亚结构及其与低密度脂蛋白的相互作用。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
低密度脂蛋白(LDL)在内皮下空间的内流和滞留是动脉粥样硬化的早期事件之一。最初,LDL必须穿过内皮糖萼,这在阻止LDL渗透中起着至关重要的作用。然而,糖萼的精确亚结构及其工作机制尚不清楚。本文通过高压冷冻/冷冻取代透射电镜显示,大鼠主动脉腔表面保存完好的多孔网状糖萼显示出三种亚型。数学模型表明,致密的下糖萼(0.2 ~ 2.9 μm)与微血管中的排列相似,LDL的分配系数为0。另一个稀疏的较高的(0.8 ~ 17.3 μm)有助于机械传导。LDL亲和柱层析结合蛋白质组学分析、共定位分析和细胞运输实验首次验证了糖萼在体外和体内都能结合LDL,但不保留LDL。小鼠耳小动脉的双光子激光扫描显微成像表明,低密度脂蛋白与糖萼之间的静电排斥相对于结合起主导作用。这些发现揭示了密集的低糖萼的排列及其对LDL的静电排斥作用,可以阻止LDL的渗透。本文章由计算机程序翻译,如有差异,请以英文原文为准。
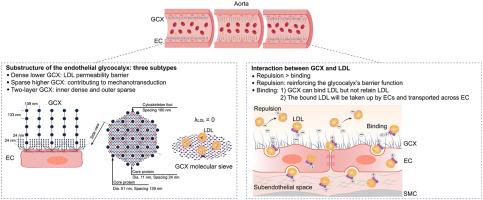
The Substructure of the Endothelial Glycocalyx in Rat Aorta and Its Interaction with the Low-Density Lipoproteins
The influx and retention of the low-density lipoproteins (LDLs) in the subendothelial space are one of the early events of atherosclerosis. Initially, LDLs must traverse the endothelial glycocalyx, which is increasingly recognized for its critical role in preventing LDL penetration. However, the precise substructure of the glycocalyx and its working mechanism are still unknown. Herein, a well-preserved porous mesh-like glycocalyx at the luminal surface of rat aortas, demonstrated by high-pressure freezing/freeze substitution transmission electron microscopy, shows three subtypes. Mathematical modeling suggests the dense lower glycocalyx (0.2 to 2.9 μm) shows similar arrangement to that reported in microvessels, with the partition coefficient of LDL equaling 0. The other sparse higher one (0.8 to 17.3 μm) contributes to mechanotransduction. LDL affinity column chromatography combined with proteomic analysis, colocalization analysis, and cell transport experiments verifies, for the first time, that the glycocalyx does bind LDLs both in vitro and in vivo, but does not retain LDLs. Two-photon laser scanning microscopic imaging of mouse ear arterioles suggests that the electrostatic repulsion between LDL and glycocalyx is dominant relative to binding. These findings reveal the arrangement of dense lower glycocalyx together with its electrostatic repulsion toward LDLs works in preventing LDL penetration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


