没食子酸酯对5α-还原酶1型的影响:神经精神疾病中神经类固醇平衡的构效关系及意义
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
神经甾体调节神经功能,其合成依赖于5α- 1型还原酶(SRD5A1)。SRD5A1的失调与神经精神疾病有关。没食子酸酯是天然存在的多酚类化合物,可以调节神经甾体生成。本研究旨在评价没食子酸和8种没食子酸酯对人和大鼠SRD5A1活性的影响,并表征它们的构效关系。利用人SF126胶质母细胞瘤细胞微粒体和大鼠脑微粒体,我们发现抑制效能随着碳链长度从丙基(C3)到十二基(C12)没食子酸酯的增加而增加(IC50值为168.29 ~ 20.53 μM),而随着碳链长度从十六基(C16)没食子酸酯的增加而降低。酶动力学分析显示混合/非竞争性抑制。与人类SRD5A1相比,大鼠SRD5A1对短链没食子酸盐的敏感性降低。在完整的细胞中,所有活性没食子酸盐都能显著减少双氢睾酮的产生。分子对接模拟表明,没食子酸盐与NADPH结合位点结合,结合能与抑制效力相关(−5.99 ~−6.61 kcal/mol)。没食子酸十六酯的C16链延伸到最佳结合区域之外,解释了其在100 μM下无效的原因。3D-QSAR模型确定疏水特征是抑制的关键,而相关分析证实了抑制效力与碳长度(C3-C12)和疏水性质之间的关系。这些发现为开发选择性SRD5A1调节剂在神经精神疾病中的潜在应用提供了结构见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
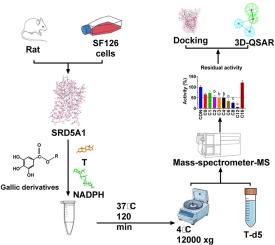
Effects of gallates on 5α-reductase type 1: structure-activity relationship and implications for neurosteroid balance in neuropsychiatric disorders
Neurosteroids modulate neural function, with their synthesis dependent on 5α-reductase type 1 (SRD5A1). Dysregulation of SRD5A1 has been implicated in neuropsychiatric disorders. Gallates, naturally occurring polyphenolic compounds, may modulate neurosteroidogenesis. This study aimed to evaluate the effects of gallic acid and eight gallate esters on human and rat SRD5A1 activity and characterize their structure-activity relationship. Using human SF126 glioblastoma cell microsomes and rat brain microsomes, we found inhibitory potency increased with carbon chain length from propyl (C3) to dodecyl (C12) gallate (IC50 values: 168.29 to 20.53 μM), but diminished with cetyl (C16) gallate. Enzyme kinetic analyses revealed mixed/noncompetitive inhibition. Rat SRD5A1 showed reduced sensitivity to shorter-chain gallates compared to human SRD5A1. In intact cells, all active gallates significantly reduced dihydrotestosterone production. Molecular docking simulations showed gallates bind to the NADPH binding site, with binding energies correlating with inhibitory potency (−5.99 to −6.61 kcal/mol). The C16 chain of cetyl gallate extended beyond the optimal binding region, explaining its ineffectiveness at 100 μM. 3D-QSAR modeling identified hydrophobic features as critical for inhibition, while correlation analyses confirmed relationships between inhibitory potency and carbon length (C3–C12) and hydrophobicity properties. These findings provide structural insights for developing selective SRD5A1 modulators with potential applications in neuropsychiatric disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


