催化天然环脱水的Azomycin生物合成酶RohQ的结构和机理。
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
来自azomycin生物合成途径的RohQ催化自发环脱水生成2-氨基咪唑。在这里,我们报道了RohQ的结构和机制,并使用偶然结合的咪唑来确定活性位点残基。我们提出,催化作用发生在二聚体界面,使用两个关键的天冬氨酸残基进行质子转移步骤,以加速胍基和醛的3 × 104倍分子内环化,释放水。我们的工作扩展了我们对新出现的酶的理解,并提供了一个尚未探索的蛋白质家族的第一个结构和机制视图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
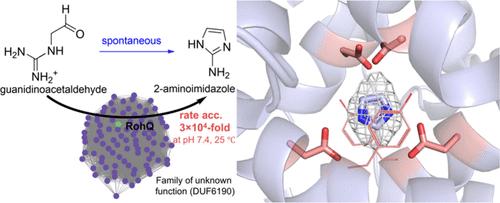
Structure and Mechanism of the Azomycin Biosynthetic Enzyme RohQ That Catalyzes a Spontaneous Cyclodehydration
RohQ from the azomycin biosynthetic pathway catalyzes a spontaneous cyclodehydration to form 2-aminoimidazole. Here we report the structure and mechanism of RohQ and use a serendipitously bound imidazole to pinpoint active site residues. We propose that catalysis occurs at the dimeric interface using two key aspartic acid residues for proton transfer steps to accelerate 3 × 104-fold intramolecular cyclization of a guanidino group and aldehyde, releasing water. Our work expands our understanding of de novo emerged enzymes and provides the first structural and mechanistic view of a yet-unexplored protein family.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


