在U2AF1S34F突变型骨髓增生异常肿瘤中,CHEK1的异常剪接是巨核细胞异常增生的驱动因素
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
U2AF1突变在骨髓增生异常肿瘤(MDS)患者中很常见,这表明由突变的U2AF1驱动的前mrna的异常剪接可能在MDS的发病机制中起关键作用。先前的研究表明,U2AF1S34F突变会损害红细胞和粒细胞的分化,但对巨核细胞(MKs)的影响尚不清楚。在这里,通过整合MDS患者和U2AF1突变细胞系的数据,我们确定U2AF1突变与巨核生成异常相关,诱导异常mk的产生,特别是微量mk,并诱导显著的血小板减少。我们确定突变体u2af1介导的DNA生物合成相关基因(如CHEK1)的异常剪接是正常MK多倍体化所必需的。反过来,CHEK1的错误剪接导致了u2af1突变MDS患者中异常mk数量的增加。此外,U2AF1S34突变诱导CHK1缺失并激活其磷酸化,从而进一步驱动MK多倍体化和成熟的损害。因此,选择性CHK1抑制剂治疗可显著减少体外异常MK的产生。综上所述,这些发现表明U2AF1突变通过驱动CHEK1细胞周期相关基因的异常剪接诱导异常mk的产生,揭示了MDS中巨核生成异常的分子基础,并确定了MDS治疗的新潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
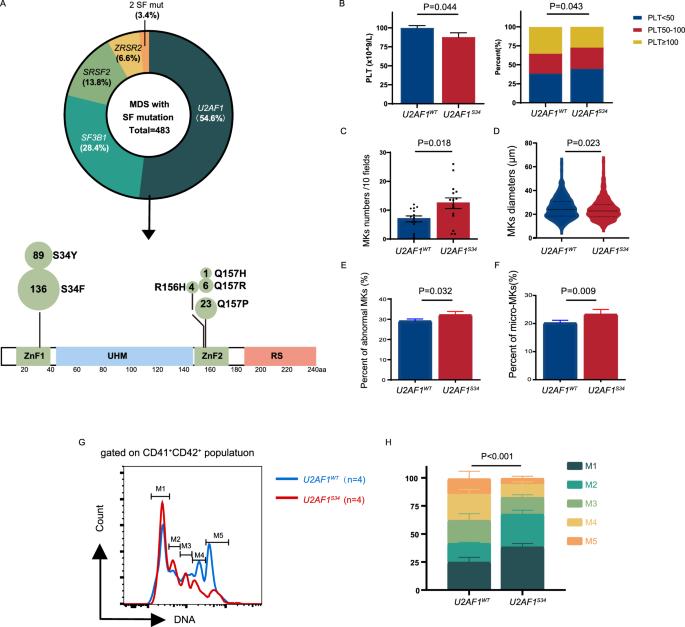

Aberrant splicing of CHEK1 is a driver of megakaryocytic dysplasia in U2AF1S34F mutant myelodysplastic neoplasms
U2AF1 mutations are common in patients with myelodysplastic neoplasms (MDS), suggesting that aberrant splicing of pre-mRNAs driven by mutant U2AF1 could play a critical role in MDS pathogenesis. Previous studies have demonstrated that U2AF1S34F mutation impairs the differentiation of erythrocytes and granulocytes, but the impact on megakaryocytes (MKs) remains unclear. Here, by integrating data from MDS patients and cell lines with U2AF1 mutations, we determined that U2AF1 mutations are associated with dysmegakaryopoiesis, induce the generation of abnormal MKs, especially micro-MKs, and induce significant thrombocytopenia. We determined that mutant U2AF1-mediated aberrant splicing of DNA biosynthesis-related genes, such as CHEK1, is required for normal MK polyploidization. The mis-splicing of CHEK1, in turn, accounts for the increased number of abnormal MKs in U2AF1-mutant MDS patients. Moreover, U2AF1S34 mutations induce the deficiency of CHK1 and the activation of its phosphorylation, thereby further driving the impairment of MK polyploidization and maturation. Accordingly, treatment with selective CHK1 inhibitor significantly reduces abnormal MK production in vitro. Taken together, these findings demonstrate that U2AF1 mutations induce the generation of abnormal MKs by driving aberrant splicing of the CHEK1 cell cycle-related gene, revealing the molecular basis for dysmegakaryopoiesis in MDS and identifying a new potential target for MDS treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


