内皮细胞STING调节il - 6的产生,是实验性心力衰竭病理性心肌肥大和收缩功能障碍所必需的。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
毛细血管稀疏和心肌细胞(CM)肥大是心力衰竭(HF)复杂综合征的标志,是住院和死亡的主要原因。心脏细胞成分内部和之间的分子信号已经成为调节心脏病理生理的中心枢纽。在这里,我们发现干扰素基因刺激因子(STING)在人和小鼠心脏内皮细胞(EC)中高度表达,并在HF发病时被激活。我们使用全局的和可诱导的EC特异性STING-/-小鼠,报告了EC STING通过调节CM肥大和毛细血管狭窄,在横断主动脉收缩(TAC)引起的HF实验模型中是必需的。我们报道,与对照组相比,从TAC EC-STING-/-小鼠中分离的ECs富含与整合素和细胞粘附细胞外基质(ECM)相关的基因集。通过对非缺血性心肌病患者心肌细胞的CellChat分析,我们发现EC-CM之间存在通信,并且在机制上,我们发现STING激活诱导il - 6分泌。此外,ec来源的il - 6以STING依赖的方式在CMs中诱导促肥厚基因表达是必要的。我们的研究表明,STING在实验性心衰的ECs肥厚和收缩功能障碍中发挥了新的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
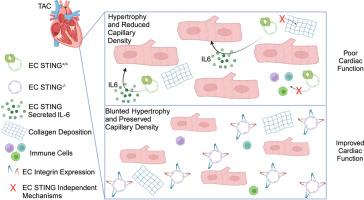
Endothelial Cell Stimulator of Interferon Genes Regulates IL-6 Production and Is Required for Pathologic Cardiac Hypertrophy and Contractile Dysfunction in Experimental Heart Failure
Capillary rarefaction and cardiomyocyte (CM) hypertrophy are hallmarks of the complex syndrome of heart failure (HF), a leading cause of hospitalization and death. Molecular signals within and between cellular components of the heart have emerged as central hubs that modulate cardiac pathophysiology. The stimulator of interferon genes (STING) was highly expressed in human and mouse cardiac endothelial cells (ECs) and was activated at the onset of HF. Global and inducible EC-specific STING−/− mice were used to demonstrate that EC STING is required for contractile dysfunction in an experimental model of HF induced by transverse aortic constriction, through the regulation of CM hypertrophy and capillary rarefaction. ECs from EC-STING−/− mice subjected to transverse aortic constriction were enriched in gene sets related to integrin and cell adhesion to extracellular matrix compared with controls. CellChat analysis of human cardiac cells from patients with nonischemic cardiomyopathy revealed EC-CM communication, and mechanistically, STING activation induced IL-6 secretion. Furthermore, EC-derived IL-6 was necessary to induce prohypertrophic gene expression in CMs in a STING-dependent manner. The study demonstrates a novel role for STING in ECs, contributing to hypertrophy and contractile dysfunction in experimental HF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


