个体特异性诱导KMT2A:: aff1融合驱动CD24明显阳性的白血病前期状态的发展
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
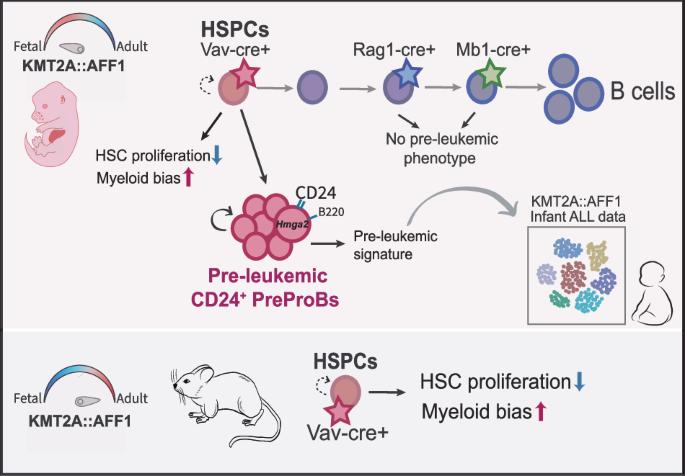
由KMT2A::AFF1肿瘤融合驱动的婴儿急性淋巴细胞白血病(ALL)是一种侵袭性、预后差的疾病,很少有合作突变。融合起源于子宫,但疾病发展的胚胎启动步骤仍然知之甚少。在这里,我们提出了一种新的小鼠KMT2A::AFF1模型,该模型提供了与人类疾病相关的KMT2A::AFF1白血病前期的关键见解。该模型能够精确地诱导癌基因,并且在靶向造血干细胞和祖细胞(HSPCs)后,观察到对造血干细胞(hsc)增殖的选择性负面影响,无论诱导过程中的发育状态如何。然而,一个独特的CD24+PreProB亚群只在KMT2A::AFF1胚胎环境中扩展。当靶向淋巴样祖细胞时,这个群体不存在,突出了起源细胞在白血病发展中的重要性。CD24+PreProB亚群显示出白血病前期干细胞的关键特征,包括谱系可塑性和异常植入能力。与它们的癌前表型一致,单细胞转录组学揭示了与干性一致的特征,以及显著的Hmga2上调,Hmga2是自我更新的调节因子。该特征可严重转移到人类KMT2A::AFF1患者。此外,考虑到CD24是一个潜在的治疗靶点,我们的研究结果揭示了一种与人类疾病直接相关的独特的胚胎白血病前期状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Ontogeny-specific induction of the KMT2A::AFF1-fusion drives development of a distinct CD24 positive pre-leukemic state
Infant Acute Lymphoblastic Leukemia (ALL) driven by the KMT2A::AFF1 onco-fusion is an aggressive, poor prognosis disease with few co-operative mutations. The fusion originates in utero, yet the embryonic initiating steps of disease development remain poorly understood. Here, we present a novel murine KMT2A::AFF1 model, that provides key insights into KMT2A::AFF1 pre-leukemia, relevant to human disease. The model enables precise oncogene induction, and upon targeting hematopoietic stem and progenitor cells (HSPCs) a selective negative impact on proliferation of hematopoietic stem cells (HSCs) was observed, regardless of developmental state during induction. However, a unique CD24+PreProB subset expanded exclusively within the KMT2A::AFF1 embryonic context. This population was absent when targeting lymphoid progenitors, highlighting the importance of the cell of origin for leukemic development. The CD24+PreProB subset displayed key features of pre-leukemic stem cells, including lineage plasticity and aberrant engraftment ability. In line with their pre-malignant phenotype, single-cell transcriptomics revealed a signature consistent with stemness, and notable, up-regulation of Hmga2, a regulator of self-renewal. The signature was critically transferable to human KMT2A::AFF1 patients. Furthermore, given that CD24 is a potential therapeutic target, our findings uncover a distinct embryonic pre-leukemic state with direct relevance to human disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


