酵母己糖激酶的铅失活及其镁的还原可能是铅毒性的一个方面
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
流行病学研究表明血铅水平与空腹血糖水平(BGL)呈正相关。葡萄糖稳态与铅暴露之间的关系尚不清楚。BGL是涉及多种酶的多种过程的结果。细胞葡萄糖代谢的关键初始步骤之一是由ATP磷酸化,在肝细胞中由己糖激酶(HK)或葡萄糖激酶催化。这种磷酸化阻止了细胞内的葡萄糖向外扩散,并使葡萄糖进一步在细胞内加工。本研究的目的是分析铅和其他二价金属对己糖激酶(HK)酶活性的影响,以了解铅对Mg2+依赖性酶的毒性作用。在Mg存在时HK活性最高,而Pb不存在时HK活性最高。我们的研究结果表明,铅以一种时间依赖性的方式抑制HK,因此需要几分钟的铅预孵育,但不含葡萄糖。一种改进的非竞争性抑制模型最能描述这种活动的丧失。Mg是唯一能部分逆转铅依赖性HK活性损失的金属。这些结果表明,铅形成稳定的金属配合物,与不同的蛋白质靶点相互作用,其中一些取代Mg,另一些在没有葡萄糖的情况下以蛋白质构象存在。在理论DFT水平上计算的数据表明,所有金属- atp复合物都促进葡萄糖磷酸化。抑制作用似乎并不依赖于PbATP复合物的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
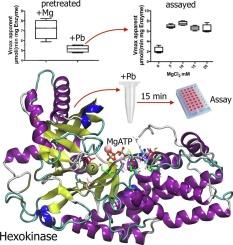
Yeast hexokinase inactivation by lead and its reversion by magnesium as one possible aspect of lead toxicity
Epidemiological studies suggest a positive association between blood lead levels and fasting blood glucose levels (BGL). The link between glucose homeostasis and Pb exposure is unclear. BGL is the result of multiple processes involving many enzymes. One of the critical initial steps in cellular glucose metabolism is its phosphorylation by ATP, catalyzed by hexokinase (HK) or glucokinase in hepatocytes. This phosphorylation prevents glucose inside cells from diffusing back out and commits glucose to further intracellular processing.
The aim of this study is to analyze the effect of lead and other divalent metals in hexokinase (HK) enzyme activity in vitro to understand the toxic effect of lead on a Mg2+-dependent enzyme. The highest HK activity was observed in the presence of Mg in absence of Pb.
Our results show that lead inhibits HK in a time-dependent fashion, thus requiring several minutes of preincubation with Pb but without glucose. A modified non-competitive inhibition model is the best to describe this loss of activity. Mg is the only metal that partially reverted the lead dependent loss of HK activity. These results suggest that lead forms stable metal complexes which interact with different protein targets, some of them displace for Mg and others available in protein conformation without glucose. Data calculated at the DFT level of theory show that all Metal-ATP complexes promote the glucose phosphorylation. Inhibition does not seem to depend on the formation of a PbATP complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


