血清微泡指标在区分不同类型药物性肝损伤中的潜在应用。
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
药物性肝损伤(DILI)可能在多种药物联合治疗后发生,这给流行病学研究和临床实践中确定肝损伤的致病药物带来了困难。黄芩(Scutellaria baicalensis, SB)是一种广泛使用的中药,有报道称黄芩与抗生素合用会引起肝损伤。然而,如何诊断药物性肝损伤(SB-DILI)并区分其与抗生素性肝损伤(AILI)是现代临床实践中的重大挑战之一。目前还没有有效的指标来区分SB-DILI和AILI。因此,为了发现和验证高度相关的指标,我们对29名SB-DILI患者、28名AILI患者和33名健康志愿者(HVs)的血清微泡(mv)实施了无标记定量蛋白质组学和平行反应监测(PRM)的质谱工作流程。结果表明,血清MVs溶菌酶(LYZ)、组分4-结合蛋白α (C4BPA)和补体组分1r (C1R)联合分析的曲线下面积(AUC) ≥ 0.95,表明联合分析在这些人群中几乎完全区分了lb - dili和AILI。该研究强调了循环MV指标在区分SB-DILI和AILI方面的潜力,为DILI的临床诊断和治疗方法提供了重要意义。意义:药物性肝损伤(DILI)在临床实践中提出了重大的诊断挑战,特别是当由草药-抗生素联合治疗引起时,确定病原体至关重要但难以捉摸。本研究通过建立首个基于血清微泡(MV)的生物标志物小组(LYZ, C4BPA和C1R)来解决这一未满足的需求,该小组能够以高精度(AUC ≥ 0.95)区分黄芩诱导的DILI (sf -DILI)和抗生素诱导的DILI (AILI)。利用无标记定量蛋白质组学和平行反应监测(PRM)的严格验证,我们的工作通过证明mv衍生蛋白反映疾病特异性病理生理过程,如补体失调和免疫激活,推动了翻译蛋白质组学领域的发展。在临床上,该小组讨论了SB-DILI和AILI(发病率)的罕见性本文章由计算机程序翻译,如有差异,请以英文原文为准。
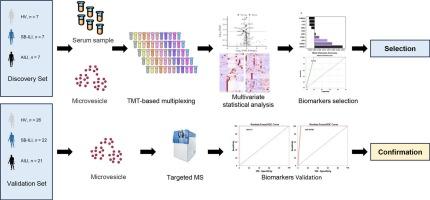
Potential use of serum microvesicle indicators to distinguish different types of drug-induced liver injury
Drug-induced liver injury (DILI) may occur after the combination therapy of multiple drugs, which makes it difficult to identify the causative drug for liver injury in epidemiological research and clinical practice. Scutellaria baicalensis (SB), a widely used herb in traditional Chinese medicine, has been reported to cause liver injury when combined with antibiotics. However, diagnosing SB-drug induced liver injury (SB-DILI) and its distinction from antibiotic-induced DILI (AILI) is one of the significant challenges in modern clinical practice. There are still no effective indicators to distinguish between SB-DILI and AILI. Therefore, to discover and validate highly relevant indicators, we implemented a mass spectrometry workflow using label-free quantitative proteomics and Parallel Reaction Monitoring (PRM) in serum microvesicles (MVs) from 29 patients with SB-DILI, 28 patients with AILI, and 33 healthy volunteers (HVs). The results showed that a combined analysis of lysozyme (LYZ), component 4-binding protein α (C4BPA), and complement component 1r (C1R) from serum MVs yielded an area under the curve (AUC) ≥ 0.95, indicating that the combination analysis nearly fully distinguished between SB-DILI and AILI in these cohorts. This study underscores the potential of circulating MV indicators in differentiating between SB-DILI and AILI, offering significant implications for clinical diagnosis and therapeutic approaches for DILI.
Significance
Drug-induced liver injury (DILI) poses significant diagnostic challenges in clinical practice, particularly when caused by herbal-antibiotic combination therapies, where identifying the causative agent is critical yet elusive. This study addresses this unmet need by establishing the first serum microvesicle (MV)-based biomarker panel (LYZ, C4BPA, and C1R) capable of distinguishing Scutellaria baicalensis-induced DILI (SB-DILI) from antibiotic-induced DILI (AILI) with high accuracy (AUC ≥ 0.95). Leveraging label-free quantitative proteomics and rigorous validation via parallel reaction monitoring (PRM), our work advances the field of translational proteomics by demonstrating that MV-derived proteins reflect disease-specific pathophysiological processes, such as complement dysregulation and immune activation.
Clinically, this panel addresses the rarity of SB-DILI and AILI (incidence <30/100,000) by providing an objective diagnostic tool to guide timely drug discontinuation, thereby reducing liver damage progression and optimizing therapeutic decisions. Methodologically, our workflow—combining MV isolation with targeted proteomics—sets a precedent for biomarker discovery in rare DILI subtypes. While preliminary, these findings lay the groundwork for multicenter validation studies to translate this innovation into clinical practice, ultimately improving precision medicine strategies for hepatotoxicity management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


