有机tin作为人与大鼠类固醇5α-还原酶1抑制剂的构效关系及对接分析
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
类固醇5α-还原酶1 (SRD5A1)催化睾酮转化为二氢睾酮,在脑和生殖系统的类固醇生成中起重要作用。本研究旨在系统评价有机素对人和大鼠SRD5A1的抑制作用及其机制。对16种有机锡进行了抑菌强度和酶抑制实验,并进行了分子对接和相关性分析。其中,二丙锡、二苯基锡、三乙基锡、三丁基锡和三苯基锡对人SRD5A1的抑制作用显著,IC50值分别为30.31、17.83、28.28、12.69和4.38 μM。二辛基锡、三丁基锡、四丙基锡和三丁基苯基锡均能显著抑制大鼠SRD5A1, IC50值在11.91 ~ 29.96 μM之间。这些有机锡对睾酮表现出混合/非竞争性抑制,并与SRD5A1的nadph结合位点结合,与关键的蛋氨酸残基形成相互作用。二硫苏糖醇部分逆转了三苯基锡的抑制作用,表明与蛋氨酸巯基的相互作用介导了抑制作用。在SF126细胞中,二苯基锡、三苯基锡和三乙基锡在≥1 μM时显著降低双氢睾酮的生成。相关分析表明,分子量、重原子和LogP与SRD5A1的抑制效能相关。这些发现阐明了有机素抑制SRD5A1的结构-活性关系和机制,强调了它们破坏类固醇生成和神经类固醇生物合成的潜力,对生殖和神经健康具有影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
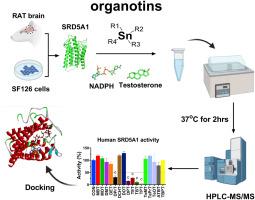
Organotins as inhibitors targeting human and rat steroid 5α-reductase 1: structure-activity relationship and docking analysis
Steroid 5α-reductase 1 (SRD5A1) catalyzes the conversion of testosterone to dihydrotestosterone, playing crucial roles in steroidogenesis in the brain and reproductive system. This study aimed to systematically evaluate the inhibitory effects and mechanisms of action of organotins on human and rat SRD5A1. Sixteen organotins were screened for the inhibitory strength and enzyme inhibition assays, molecular docking, and correlation analyses were performed. Among the tested compounds, dipropyltin, diphenyltin, triethyltin, tributyltin, and triphenyltin significantly inhibited human SRD5A1 with IC50 values of 30.31, 17.83, 28.28, 12.69, and 4.38 μM, respectively. Dioctyltin, tributyltin, tetrapropyltin, and tributylphenyltin also markedly inhibited rat SRD5A1 with IC50 values ranging from 11.91 to 29.96 μM. These organotins exhibited mixed/noncompetitive inhibition with respect to testosterone and bound to the NADPH-binding site of SRD5A1, forming interactions with critical methionine residues. Dithiothreitol partially reversed inhibition by triphenyltin, suggesting that interaction with the sulfhydryl group of methionine mediates inhibition. In SF126 cells, diphenyltin, triphenyltin, and triethyltin significantly reduced dihydrotestosterone production at ≥ 1 μM. Correlation analysis indicated that molecular weight, heavy atoms, and LogP were correlated with inhibitory potency against SRD5A1. These findings elucidate the structure-activity relationships and mechanisms by which organotins inhibit SRD5A1, highlighting their potential to disrupt steroidogenesis and neurosteroid biosynthesis, with implications for reproductive and neurological health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


