CuCeZrO2-δ催化剂烟尘氧化活性及抗硫中毒性能的实验研究
IF 4.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
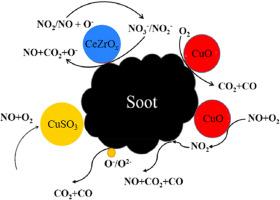
本文采用SHS法制备了CuCeZrO2-δ烟灰氧化催化剂。同时,通过硫处理和在含SO2气氛下的反应,评价了该系列催化剂的抗硫性能,并通过TEM、XPS、XRD、N2等温吸附解吸、in situ DRIFTS等手段对催化剂进行了表征。CeZrO2-δ上负载Cu能在一定程度上促进烟尘燃烧,提高CO2选择性,其综合性能优于Pt贵金属催化剂。这是因为Cu负载可以使晶粒更加分散,促进NO在催化剂表面的反应生成NO2。CuCeZrO2-δ的整体抗硫性能优于Pt催化剂。硫中毒降低了催化剂颗粒的分散性,显著降低了催化剂表面吸附的活性氧OA,导致催化剂表面NO氧化为NO2的过程速率降低。与CuO和CeZrO2-δ催化剂相比,CuCeZrO2-δ具有较强的NO氧化能力和优异的NO2存储能力,CuO和CeZrO2-δ具有较强的协同效应,大大提高了各自的优势。硫中毒后CuCeZrO2-δ催化剂的吸附能力不受影响。催化剂失活主要是由于Cu元素中毒,削弱了催化剂氧化NO的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental study on the soot oxidation activity and sulfur poisoning resistance of CuCeZrO2-δ catalyst
In this paper, CuCeZrO2-δ catalyst for soot oxidation was prepared by SHS method. At the same time, through sulfur-processing and reaction in the atmosphere containing SO2, the sulfur resistance of this series of catalysts was evaluated, and the catalysts were characterized by TEM, XPS, XRD, N2 isothermal adsorption desorption, In situ DRIFTS, etc. Cu loading on CeZrO2-δ can promote soot combustion and improve CO2 selectivity to a certain extent, and its overall performance is better than Pt noble metal catalyst. This is because Cu loading can make crystal grains more dispersed, and promote the reaction of NO to generate NO2 on the catalyst surface. The overall sulfur resistance of CuCeZrO2-δ is better than that of Pt catalyst. Sulfur poisoning reduced the dispersion of catalyst grains, significantly reduced the active oxygen OA adsorbed on the catalyst surface, and led to the reduction of the process rate of NO oxidation to NO2 on the catalyst surface. Compared with CuO and CeZrO2-δ catalysts, CuCeZrO2-δ has both strong NO oxidation capacity and excellent NO2 storage capacity, and CuO and CeZrO2-δ have a strong synergistic effect, which greatly improves their respective advantages. The adsorption capacity of CuCeZrO2-δ catalyst after sulfur poisoning was not affected. The deactivation of the catalyst is mainly due to the poisoning of Cu element, which weakens the ability of the catalyst to oxidize NO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


